Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00136
|
|||||
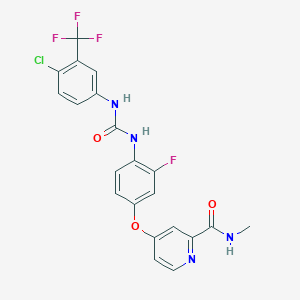
| Drug Name |
Regorafenib
|
|||||
| Synonyms |
24T2A1DOYB; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; BAY73-4506; BAY73-4506 hydrochloride; CHEBI:68647; CHEMBL1946170; Regorafenib (BAY 73-4506); Regorafenib [USAN:INN]; Regorafenibum; Stivarga; Stivarga (TN); UNII-24T2A1DOYB
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Metastatic colorectal cancer [ICD11: 2B91] | Approved | [1] | |||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H15ClF4N4O3
|
|||||
| Canonical SMILES |
CNC(=O)C1=NC=CC(=C1)OC2=CC(=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F)F
|
|||||
| InChI |
InChI=1S/C21H15ClF4N4O3/c1-27-19(31)18-10-13(6-7-28-18)33-12-3-5-17(16(23)9-12)30-20(32)29-11-2-4-15(22)14(8-11)21(24,25)26/h2-10H,1H3,(H,27,31)(H2,29,30,32)
|
|||||
| InChIKey |
FNHKPVJBJVTLMP-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 755037-03-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 482.8 | Topological Polar Surface Area | 92.4 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
4.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
123055398
, 124757057
, 124772081
, 125163861
, 126731249
, 131480720
, 135261819
, 135626856
, 135727382
, 136367351
, 136368060
, 136920288
, 136937804
, 136967459
, 137245314
, 137275982
, 142611634
, 144115687
, 152090526
, 152258021
, 152258178
, 152344131
, 160645781
, 160647014
, 162011543
, 162037486
, 16247148
, 163698233
, 164041884
, 164764796
, 172087026
, 172093559
, 174530361
, 175267155
, 175427139
, 178102514
, 186014501
, 188899566
, 198992717
, 223381220
, 223498454
, 223565986
, 223680722
, 223704854
, 223925935
, 226756197
, 23360295
, 42222352
, 75430857
, 99437016
|
|||||
| ChEBI ID |
ChEBI:68647
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Regorafenib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017 Jan 4;45(D1):D353-D361. (dg:DG01913) | |||||
| 3 | Regorafenib is transported by the organic anion transporter 1B1 and the multidrug resistance protein 2. Biol Pharm Bull. 2015;38(4):582-6. | |||||
| 4 | Brain and Testis Accumulation of Regorafenib is Restricted by Breast Cancer Resistance Protein (BCRP/ABCG2) and P-glycoprotein (P-GP/ABCB1). Pharm Res. 2015 Jul;32(7):2205-16. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
