Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00153
|
|||||
| Drug Name |
Fluorouracil
|
|||||
| Synonyms |
1-fluoro-1h-pyrimidine-2,4-dione; 2,4-Dihydroxy-5-fluoropyrimidine; 2,4-Dioxo-5-fluoropryimidine; 2,4-Dioxo-5-fluoropyrimidine; 5 FU Lederle; 5 FU medac; 5 Fluorouracil; 5 Fluorouracil biosyn; 5 HU Hexal; 5-FU; 5-FU (TN); 5-FU Lederle; 5-FU medac; 5-Faracil; 5-Fluor-2,4(1H,3H)-pyrimidindion; 5-Fluor-2,4(1H,3H)-pyrimidindion [Czech]; 5-Fluor-2,4-dihydroxypyrimidin; 5-Fluor-2,4-dihydroxypyrimidin [Czech]; 5-Fluor-2,4-pyrimidindiol; 5-Fluor-2,4-pyrimidindiol [Czech]; 5-Fluoracil; 5-Fluoracil [German]; 5-Fluoracyl; 5-Fluoro-2,4(1H,3H)-pyrimidinedione; 5-Fluoro-2,4-pyrimidinedione; 5-Fluoropyrimidin-2,4-diol; 5-Fluoropyrimidine-2,4-dione; 5-Fluorouracil; 5-Fluorouracil-biosyn; 5-Fluoruracil; 5-Fluoruracil [German]; 5-Ftouracyl; 5-HU Hexal; 5-fluoro uracil; 5-fluoro-1H-pyrimidine-2,4-dione; 5-fluoropyrimidine-2,4(1H,3H)-dione; 5FU; Adrucil; Adrucil (TN); Allergan Brand of Fluorouracil; Arumel; Biosyn Brand of Fluorouracil; CSP Brand of Fluorouracil; Carac; Carac (TN); Carzonal; Cinco FU; Dakota Brand of Fluorouracil; Dakota, Fluorouracile; Dermatech Brand of Fluorouracil; Dermik Brandof Fluorouracil; Effluderm; Effluderm (free base); Efudex; Efudex (TN); Efudix; Efurix; F 6627; F0151; Ferrer Brand of Fluorouracil; Fluoro Uracile ICN; Fluoro-Uracile ICN; Fluoro-uracile; Fluoro-uracilo; Fluoroblastin; Fluoroplex; Fluoroplex (TN); Fluorouracil (JP15/USP/INN); Fluorouracil GRY; Fluorouracil Mononitrate; Fluorouracil Monopotassium Salt; Fluorouracil Monosodium Salt; Fluorouracil Potassium Salt; Fluorouracil Teva Brand; Fluorouracil [USAN:INN:BAN:JAN]; Fluorouracil-GRY; Fluorouracile; Fluorouracile Dakota; Fluorouracile [DCIT]; Fluorouracilo; Fluorouracilo Ferrer Far; Fluorouracilo [INN-Spanish]; Fluorouracilum; Fluorouracilum [INN-Latin]; Fluoruracil; Fluracedyl; Fluracil; Fluracilum; Fluri; Fluril; Fluro Uracil; Fluroblastin; Flurodex; Ftoruracil; Gry Brand of Fluorouracil; Haemato Brand of Fluorouracil; Haemato fu; Haemato-fu; Hexal Brand of Fluorouracil; ICN Brand of Fluorouracil; IN1335; Inhibits thymilidate synthetase; Kecimeton; Medac Brand of Fluorouracil; Neocorp Brand of Fluorouracil; Neofluor; Onkofluor; Onkoworks Brand of Fluorouracil; Phthoruracil; Phtoruracil; Queroplex; Ribofluor; Ribosepharm Brand of Fluorouracil; Riemser Brand of Fluorouracil; Ro 2-9757; Ro-2-9757; Roche Brand of Fluorouracil; Tetratogen; Teva Brand of Fluorouracil; Timazin; U 8953; U-8953; URF; Ulup
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Colon cancer [ICD11: 2B90.Z] | Approved | [1] | |||
| Esophageal cancer [ICD11: 2B70] | Approved | [1] | ||||
| Stomach cancer [ICD11: 2B72] | Approved | [1] | ||||
| Pancreatic cancer [ICD11: 2C10] | Approved | [1] | ||||
| Therapeutic Class |
Immunosuppressive Agents
|
|||||
| Structure |
|
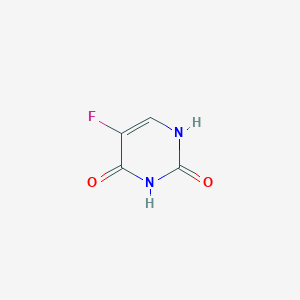 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C4H3FN2O2
|
|||||
| Canonical SMILES |
C1=C(C(=O)NC(=O)N1)F
|
|||||
| InChI |
InChI=1S/C4H3FN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
|
|||||
| InChIKey |
GHASVSINZRGABV-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 51-21-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 130.08 | Topological Polar Surface Area | 58.2 | ||
| Heavy Atom Count | 9 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10524722
, 11111190
, 11335229
, 11360468
, 11363735
, 11366297
, 11368859
, 11371368
, 11374392
, 11377021
, 11406045
, 11461440
, 11484027
, 11487892
, 11490250
, 11492372
, 11494655
, 11538022
, 15218968
, 17389875
, 17405099
, 22391543
, 24276773
, 24278439
, 24871165
, 24894963
, 25346604
, 25621761
, 26611750
, 26679238
, 26697058
, 26747342
, 26747343
, 26747344
, 26752979
, 26758708
, 3139714
, 5132902
, 5367838
, 595836
, 603131
, 7847650
, 7891022
, 7978600
, 8139872
, 8149350
, 8152156
, 82653
, 841046
, 9851
|
|||||
| ChEBI ID |
ChEBI:46345
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| ENT1 | Transporter Info | Equilibrative nucleoside transporter 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [5] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [5] | ||
| MRP5 | Transporter Info | Multidrug resistance-associated protein 5 | Substrate | [5] | ||
| OAT2 | Transporter Info | Organic anion transporter 2 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [7] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT2 | Transporter Info | Km = 0.0538 microM | Oocytes-OAT2 | [6] | |
| References | ||||||
| 1 | Fluorouracil was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Role of BCRP as a biomarker for predicting resistance to 5-fluorouracil in breast cancer. Cancer Chemother Pharmacol. 2009 May;63(6):1103-10. | |||||
| 3 | Human equilibrative nucleoside transporter 1, as a predictor of 5-fluorouracil resistance in human pancreatic cancer. Anticancer Res. 2007 Jul-Aug;27(4B):2241-9. | |||||
| 4 | Enhancing chemosensitivity in oral squamous cell carcinoma by lentivirus vector-mediated RNA interference targeting EGFR and MRP2. Oncol Lett. 2016 Sep;12(3):2107-2114. | |||||
| 5 | ATP-binding cassette C transporters in human pancreatic carcinoma cell lines. Upregulation in 5-fluorouracil-resistant cells. Pancreatology. 2009;9(1-2):136-44. | |||||
| 6 | Transport mechanism and substrate specificity of human organic anion transporter 2 (hOat2 [SLC22A7]). J Pharm Pharmacol. 2005 May;57(5):573-8. | |||||
| 7 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
