Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00399
|
|||||
| Drug Name |
Valproic Acid
|
|||||
| Synonyms |
2 PP (base); 2-Propylpentanoic acid; 2-Propylvaleric acid; 2-n-Propyl-n-valeric acid; 4-Heptanecarboxylic acid; Abbott 44090; Acide valproique; Acide valproique [INN-French]; Acido valproico; Acido valproico [INN-Spanish]; Acidum valproicum; Acidum valproicum [INN-Latin]; Alti-Valproic; Avugane; Baceca; Convulex; Convulex (TN); Convulsofin; Depacon (TN); Depakene; Depakene (TN); Depakin; Depakin chrono; Depakine; Depakine chrono; Depakote (TM); Depakote (TN); Depakote ER (TN); Deproic; Di-n-propylacetic acid; Di-n-propylessigsaeure; Di-n-propylessigsaure; Di-n-propylessigsaure [German]; Dipropyl Acetate; Dipropylacetate; Dipropylacetic acid; Dom-Valproic; Encorate (TN); Epilim (TN); Epival (TN); Ergenyl; G2M-777; Kyselina 2-propylvalerova; Kyselina 2-propylvalerova [Czech]; Med Valproic; Mylproin; Myproic Acid; N-DPA; N-Dipropylacetic acid; Novo-Valproic; Nu-Valproic; PMS-Valproic Acid; Penta-Valproic; Propylvaleric acid; Savicol; Semisodium Valproate; Stavzor; Stavzor (TN); VPA; Valproate; Valproic Acid, Sodium Salt (2:1); Valproic acid (USP); Valproic acid USP; Valproic acid USP24; Valproic acid [USAN:INN:BAN]; Valproinsaeure; Vupral; Winthrop (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Epilepsy [ICD11: 8A6Z] | Approved | [1] | |||
| Therapeutic Class |
Anticonvulsants
|
|||||
| Structure |
|
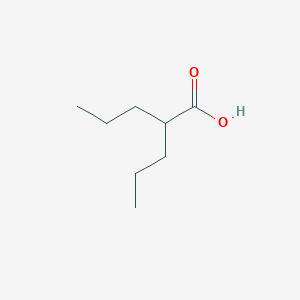 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H16O2
|
|||||
| Canonical SMILES |
CCCC(CCC)C(=O)O
|
|||||
| InChI |
InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10)
|
|||||
| InChIKey |
NIJJYAXOARWZEE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 99-66-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 144.21 | Topological Polar Surface Area | 37.3 | ||
| Heavy Atom Count | 10 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
2.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10539166
, 11335448
, 11360687
, 11363415
, 11364691
, 11365977
, 11367253
, 11368539
, 11369815
, 11372725
, 11372856
, 11374010
, 11375415
, 11376701
, 11377978
, 11461659
, 11484648
, 11488762
, 11491548
, 11492191
, 11494335
, 12015354
, 14710660
, 15321539
, 17389523
, 24898751
, 26697333
, 26752920
, 26752921
, 29222263
, 3138784
, 399407
, 46260925
, 46505925
, 48416692
, 48424277
, 48425730
, 4918409
, 49635685
, 49640649
, 49856166
, 50062089
, 50105536
, 53788878
, 56310655
, 621684
, 7847465
, 7980632
, 8151988
, 9394
|
|||||
| ChEBI ID |
ChEBI:39867
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | MCT2 | Transporter Info | Monocarboxylate transporter 2 | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Valproic Acid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Monocarboxylate Transporters in Drug Disposition: Role in the Toxicokinetics and Toxicodynamics of the Drug of Abuse GHB. | |||||
| 3 | Pharmacogenetics of membrane transporters: an update on current approaches. Mol Biotechnol. 2010 Feb;44(2):152-67. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
