Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01275
|
|||||
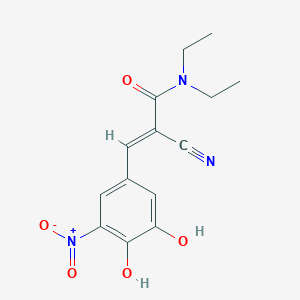
| Drug Name |
Entacapone
|
|||||
| Synonyms |
(2E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylprop-2-enamide; (E)-2-Cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethyl-2-propenamide; (E)-2-cyano-3-(3,4-dihydroxy-5-nitro-phenyl)-N,N-diethyl-prop-2-enamide; (E)-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)-N,N-diethylprop-2-enamide; (E)-alpha-Cyano-N,N-diethyl-3,4-dihydroxy-5-nitrocinnamamide; 2-Cyano-N,N-diethyl-3-(3,4-dihydroxy-5-nitrophenyl)propenamide; COM-998; Comtan; Comtan (TN); Comtess; Entacapona; Entacapona [INN-Spanish]; Entacapone (JAN/USAN/INN); Entacapone [USAN:INN]; Entacaponum; Entacaponum [INN-Latin]; KB475572; N,N-diethyl-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl) acrylamide; Novartis brand of entacapone; OR 611; OR-611; Orion brand of entacapone; Stalevo (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Parkinson's Disease [ICD11: 8A00.0] | Approved | [1] | |||
| Therapeutic Class |
Antiparkinson Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C14H15N3O5
|
|||||
| Canonical SMILES |
CCN(CC)C(=O)C(=CC1=CC(=C(C(=C1)O)O)[N+](=O)[O-])C#N
|
|||||
| InChI |
InChI=1S/C14H15N3O5/c1-3-16(4-2)14(20)10(8-15)5-9-6-11(17(21)22)13(19)12(18)7-9/h5-7,18-19H,3-4H2,1-2H3/b10-5+
|
|||||
| InChIKey |
JRURYQJSLYLRLN-BJMVGYQFSA-N
|
|||||
| CAS Number |
CAS 130929-57-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 305.29 | Topological Polar Surface Area | 130 | ||
| Heavy Atom Count | 22 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
2.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10145
, 7847846
, 7979169
, 8616509
, 11528716
, 12014660
, 14776335
, 26757974
, 39290001
, 46508734
, 49658624
, 50064207
, 50214283
, 53790856
, 57358005
, 92308972
, 92719407
, 93166184
, 103248957
, 104004044
, 113854525
, 119504915
, 121682934
, 124893160
, 126530712
, 126592033
, 126620827
, 126653084
, 126666453
, 131297374
, 134337899
, 135017133
, 135032923
, 137001453
, 141610450
, 144115941
, 144205737
, 152104428
, 160963840
, 163620784
, 163686109
, 164814929
, 164840953
, 170464850
, 172917348
, 175268297
, 175610943
, 176484081
, 177748942
, 178103260
|
|||||
| ChEBI ID |
ChEBI:4798
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| References | ||||||
| 1 | Entacapone was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Elucidation of the Impact of P-glycoprotein and Breast Cancer Resistance Protein on the Brain Distribution of Catechol-O-Methyltransferase Inhibitors. Drug Metab Dispos. 2017 Dec;45(12):1282-1291. | |||||
| 3 | Organic Anion Transporter 2-Mediated Hepatic Uptake Contributes to the Clearance of High-Permeability-Low-Molecular-Weight Acid and Zwitterion Drugs: Evaluation Using 25 Drugs. J Pharmacol Exp Ther. 2018 Nov;367(2):322-334. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
