Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00032
|
|||||
| Drug Name |
Imatinib
|
|||||
| Synonyms |
112GI019; 4-(4-METHYL-PIPERAZIN-1-YLMETHYL)-N-[4-METHYL-3-(4-PYRIDIN-3-YL-PYRIMIDIN-2-YLAMINO)-PHENYL]-BENZAMIDE; 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide; 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-benzamide methanesulfonate; 4-[(4-methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide; 4-[(4-methylpiperazin-1-yl)methyl]-N-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide; 4-[(4-methylpiperazin-1-yl)methyl]-N-{4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl}benzamide; Alpha-(4-Methyl-1-piperazinyl)-3'-((4-(3-pyridyl)-2-pyrimidinyl)amino)-p-tolu-p-toluidide; Benzamide, 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-(9CI); CGP 57148B; Cgp 57148; Glamox; Glamox (TN); Gleevec (TN); Glivec (TN); Imatinib (INN); Imatinib Methansulfonate; Imatinib [INN:BAN]; Imatinib free base; N-(3-(4-(pyridin-3-yl)pyrimidin-2-ylamino)-4-methylphenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide; STI; STI 571; STI571; Sti-571
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gastrointestinal stromal tumor [ICD11: 2B5B] | Approved | [1] | |||
| Chronic myelogenous leukemia [ICD11: 2A20.0] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
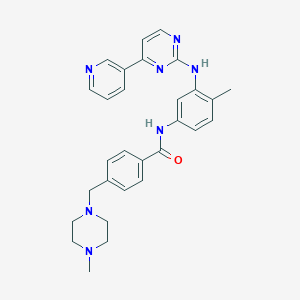 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C29H31N7O
|
|||||
| Canonical SMILES |
CC1=C(C=C(C=C1)NC(=O)C2=CC=C(C=C2)CN3CCN(CC3)C)NC4=NC=CC(=N4)C5=CN=CC=C5
|
|||||
| InChI |
InChI=1S/C29H31N7O/c1-21-5-10-25(18-27(21)34-29-31-13-11-26(33-29)24-4-3-12-30-19-24)32-28(37)23-8-6-22(7-9-23)20-36-16-14-35(2)15-17-36/h3-13,18-19H,14-17,20H2,1-2H3,(H,32,37)(H,31,33,34)
|
|||||
| InChIKey |
KTUFNOKKBVMGRW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 152459-95-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 493.6 | Topological Polar Surface Area | 86.3 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
3.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
14859628
, 22394533
, 24424247
, 26697112
, 26737110
, 29215405
, 29215406
, 29224346
, 46392211
, 46393540
, 46505055
, 46507948
, 46513933
, 49655235
, 50066026
, 50070642
, 50100104
, 50109856
, 50353059
, 53788935
, 53799240
, 5619104
, 56311252
, 56311284
, 56311359
, 56311779
, 56311988
, 56312022
, 56312838
, 56313109
, 56313183
, 56313522
, 56313562
, 56314521
, 57288246
, 57288452
, 57288559
, 57288780
, 57322698
, 57551951
, 57578266
, 584799
, 7890613
, 7979593
, 8153249
, 822644
, 828861
, 832827
, 841977
, 85171056
|
|||||
| ChEBI ID |
ChEBI:45783
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [3] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [4] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [4] | ||
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [6] | ||
| References | ||||||
| 1 | Imatinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004 Nov 1;104(9):2940-2. | |||||
| 3 | Environmental and genetic factors affecting transport of imatinib by OATP1A2. Clin Pharmacol Ther. 2011 Jun;89(6):816-20. | |||||
| 4 | Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013 Mar 15;19(6):1458-66. | |||||
| 5 | Pharmacologic markers and predictors of responses to imatinib therapy in patients with chronic myeloid leukemia. Leuk Lymphoma. 2008 Apr;49(4):639-42. | |||||
| 6 | Association of genetic polymorphisms in the influx transporter SLCO1B3 and the efflux transporter ABCB1 with imatinib pharmacokinetics in patients with chronic myeloid leukemia. Ther Drug Monit. 2011 Apr;33(2):244-50. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
