Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00050
|
|||||
| Drug Name |
Erythromycin
|
|||||
| Synonyms |
A/T/S; Abboticin; Abomacetin; Acneryne; Acnesol; Adecane-2,10-dione (non-preferred name); Ak-Mycin; Akne Cordes Losung; Akne-Mycin; Akne-mycin (TN); Aknederm Ery Gel; Aknemycin; Aknin; AustriaS; Benzamycin; Benzamycin Pak; C-Solve-2; Del-Mycin; Derimer; Deripil; Dotycin; Dumotrycin; E-Base; E-Base (base); E-Glades; E-Mycin; E-Mycin (base); E-Solve 2; E-mycin, Erycin, Robimysin; E0751; ERY; ERYC; ERYC (base); ERYTHROMYCIN STEARATE; Emgel; Emu-V; Emu-Ve; Emuvin; Emycin; Endoeritrin; Erecin; Erimycin-T; Erisone; Eritomicina; Eritrocina; Eritromicina; Eritromicina [INN-Spanish]; Ermycin; Eros; Ery-B; Ery-Diolan; Ery-Sol; Ery-Tab; Ery-Tab (base); Ery-maxin; Eryacne; Eryacnen; Eryc (TN); Eryc 125; Eryc Sprinkles; Eryc-125; Eryc-250; Erycen; Erycette; Erycin; Erycinum; Eryderm; Erydermer; Erygel; Erygel (TN); Eryhexal; Erymax; Erymed; Erysafe; Erytab; Erythra-Derm; Erythro; Erythro-Statin; Erythro-Teva; Erythroderm; Erythrogran; Erythroguent; Erythromast 36; Erythromid; Erythromycin & VRC3375; Erythromycin (JP15/USP/INN); Erythromycin A; Erythromycin A, T-Stat, Pantomicina, HSDB 3074, Erytab, DRG-0279; Erythromycin Lactate; Erythromycin Ointment; Erythromycin [INN:BAN:JAN]; Erythromycin base; Erythromycin intravenous; Erythromycin sodium lauryl sulfate; Erythromycin, compd. with monododecyl sulfate, sodium salt; Erythromycine; Erythromycine [INN-French]; Erythromycinum; Erythromycinum [INN-Latin]; Erytop; Erytrociclin; Ilocaps; Ilosone (TN); Ilosone (estolate); Iloticina; Ilotycin; Ilotycin T.S; Ilotycin T.S.; Inderm; Inderm Gel; IndermRetcin; Kesso-Mycin; Latotryd; Lederpax; Mephamycin; Mercina; N-Methylerythromycin A; Oftalmolosa Cusi Eritromicina; Oftamolets; PCE Dispertab (base); Paediathrocin; Pantoderm; Pantodrin; Pantomicina; Pce (TN); Pharyngocin; Primacine; Propiocine; Proterytrin; R-P Mycin; Retcin; Robimycin; Romycin; Sans-acne; Sansac; Skid Gel E; Staticin; Staticin (TN); Stiemicyn; Stiemycin; Sulfuric acid, monododecyl ester, sodium salt, compd. with erythromycin; T-Stat; T-stat (TN); Taimoxin-F; Theramycin Z; Tiloryth; Tiprocin; Torlamicina; Udima Ery Gel; Wemid
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Syphilis [ICD11: 1A60-1A6Z] | Approved | [1] | |||
| Chlamydia infections [ICD11: 1C2Z] | Approved | [1] | ||||
| Pelvic inflammatory disease [ICD11: GA05] | Approved | [1] | ||||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
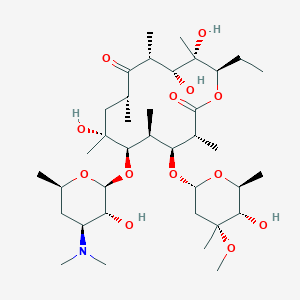 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C37H67NO13
|
|||||
| Canonical SMILES |
CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC(O3)C)N(C)C)O)(C)O)C)C)O)(C)O
|
|||||
| InChI |
InChI=1S/C37H67NO13/c1-14-25-37(10,45)30(41)20(4)27(39)18(2)16-35(8,44)32(51-34-28(40)24(38(11)12)15-19(3)47-34)21(5)29(22(6)33(43)49-25)50-26-17-36(9,46-13)31(42)23(7)48-26/h18-26,28-32,34,40-42,44-45H,14-17H2,1-13H3/t18-,19-,20+,21+,22-,23+,24+,25-,26+,28-,29+,30-,31+,32-,34+,35-,36-,37-/m1/s1
|
|||||
| InChIKey |
ULGZDMOVFRHVEP-RWJQBGPGSA-N
|
|||||
| CAS Number |
CAS 114-07-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 733.9 | Topological Polar Surface Area | 194 | ||
| Heavy Atom Count | 51 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 14 | |||
| XLogP |
2.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321305
, 11335469
, 11335572
, 11335877
, 11360708
, 11360811
, 11361116
, 11362969
, 11363553
, 11365531
, 11366115
, 11368093
, 11368677
, 11374314
, 11376255
, 11376839
, 11461680
, 11461783
, 11462088
, 11484514
, 11484962
, 11488575
, 11489079
, 11492353
, 11493929
, 11494473
, 12012581
, 14720219
, 14840178
, 14864494
, 25622201
, 26611731
, 26681121
, 29280771
, 46508487
, 47365088
, 47365089
, 47662182
, 47810649
, 47885308
, 47959639
, 5020
, 602912
, 7847208
, 7887354
, 7979183
, 8149330
, 8159405
, 823926
, 855292
|
|||||
| ChEBI ID |
ChEBI:42355
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OAT2 | Transporter Info | Organic anion transporter 2 | Substrate | [4] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [6] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OAT2 | Transporter Info | Km = 18.5 microM | Oocytes-OAT2 | [4] | |
| References | ||||||
| 1 | Erythromycin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | ABCG2: a perspective. Adv Drug Deliv Rev. 2009 Jan 31;61(1):3-13. | |||||
| 3 | Effect of ABCC2 (MRP2) transport function on erythromycin metabolism. Clin Pharmacol Ther. 2011 May;89(5):693-701. | |||||
| 4 | Possible involvement of organic anion transporter 2 on the interaction of theophylline with erythromycin in the human liver. Drug Metab Dispos. 2005 May;33(5):619-22. | |||||
| 5 | Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009 Oct;158(3):693-705. | |||||
| 6 | Enhanced corneal absorption of erythromycin by modulating P-glycoprotein and MRP mediated efflux with corticosteroids. Pharm Res. 2009 May;26(5):1270-82. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
