Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00112
|
|||||
| Drug Name |
Irinotecan
|
|||||
| Synonyms |
(+)-Irinotecan; (4S)-4,11-DIETHYL-4-HYDROXY-3,14-DIOXO-3,4,12,14-TETRAHYDRO-1H-PYRANO[3',4':6,7]INDOLIZINO[1,2-B]QUINOLIN-9-YL 1,4'-BIPIPERIDINE-1'-CARBOXYLATE; (4S)-4,11-Diethyl-4-hydroxy-3,14-dioxo-4,12-dihydro-1H-pyrano[3,4-f]quinolino[2,3-a]indolizin-9-yl 4-piperidylpiperidinecarboxylate; Biotecan; Biotecan (TN); CP0; Campto (TN); Camptosar; Camptosar (TN); Camptosar, Campto, CPT-11, Irinotecan; IRINOTECAN HYDROCHLORIDE Trihydrate; IRINOTECAN, CPT-11; Irinotecan (INN); Irinotecan (TOPO1 inhibitor); Irinotecan Hcl; Irinotecan [INN:BAN]; Irinotecan hydrochloride; Irinotecanum; Irinotecanum [INN-Latin]
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Colorectal cancer [ICD11: 2B91] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
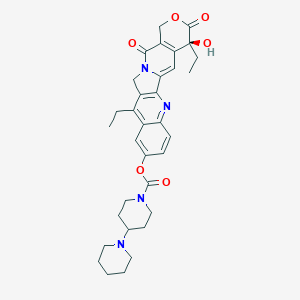 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C33H38N4O6
|
|||||
| Canonical SMILES |
CCC1=C2CN3C(=CC4=C(C3=O)COC(=O)C4(CC)O)C2=NC5=C1C=C(C=C5)OC(=O)N6CCC(CC6)N7CCCCC7
|
|||||
| InChI |
InChI=1S/C33H38N4O6/c1-3-22-23-16-21(43-32(40)36-14-10-20(11-15-36)35-12-6-5-7-13-35)8-9-27(23)34-29-24(22)18-37-28(29)17-26-25(30(37)38)19-42-31(39)33(26,41)4-2/h8-9,16-17,20,41H,3-7,10-15,18-19H2,1-2H3/t33-/m0/s1
|
|||||
| InChIKey |
UWKQSNNFCGGAFS-XIFFEERXSA-N
|
|||||
| CAS Number |
CAS 100286-90-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 586.7 | Topological Polar Surface Area | 113 | ||
| Heavy Atom Count | 43 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103169165
, 104020044
, 104253165
, 104321776
, 11409441
, 117623718
, 118049657
, 124757074
, 124893595
, 125163878
, 126630981
, 126650121
, 126663759
, 129430288
, 134337937
, 134338553
, 135032920
, 136342503
, 137001856
, 142371089
, 143493285
, 143493286
, 144116083
, 14764625
, 14911520
, 152034388
, 152240238
, 43118176
, 46393294
, 46505871
, 47811029
, 48427687
, 49835834
, 49894524
, 50422254
, 51090967
, 53788707
, 56312891
, 56314523
, 57288560
, 57314141
, 6436484
, 645391
, 7886725
, 7979640
, 81092817
, 8187089
, 85789488
, 92711320
, 96024776
|
|||||
| ChEBI ID |
ChEBI:80630
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| MRP4 | Transporter Info | Multidrug resistance-associated protein 4 | Substrate | [5] | ||
| MRP5 | Transporter Info | Multidrug resistance-associated protein 5 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP2 | Transporter Info | Km = 48.9 microM | Bile canalicular membrane vesicles-MRP2 | [7] | |
| MRP2 | Transporter Info | Km = 90.8 microM | Madin-Darby canine kidney cells (MDCKII)-MRP2 | [4] | ||
| P-GP | Transporter Info | Km = 116.1 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [4] | ||
| P-GP | Transporter Info | Km = 45.5 microM | Madin-Darby canine kidney cells (MDCKII)-MDR1 | [4] | ||
| References | ||||||
| 1 | Irinotecan was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Cyclosporin A, tacrolimus and sirolimus are potent inhibitors of the human breast cancer resistance protein (ABCG2) and reverse resistance to mitoxantrone and topotecan. Cancer Chemother Pharmacol. 2006 Sep;58(3):374-83. | |||||
| 3 | ATP-Dependent efflux of CPT-11 and SN-38 by the multidrug resistance protein (MRP) and its inhibition by PAK-104P. Mol Pharmacol. 1999 May;55(5):921-8. | |||||
| 4 | Intestinal transport of irinotecan in Caco-2 cells and MDCK II cells overexpressing efflux transporters Pgp, cMOAT, and MRP1. Drug Metab Dispos. 2002 Jul;30(7):763-70. | |||||
| 5 | P-glycoprotein, but not multidrug resistance protein 4, plays a role in the systemic clearance of irinotecan and SN-38 in mice. Drug Metab Lett. 2010 Dec;4(4):195-201. | |||||
| 6 | Celecoxib upregulates multidrug resistance proteins in colon cancer: lack of synergy with standard chemotherapy. Curr Cancer Drug Targets. 2008 Aug;8(5):414-20. | |||||
| 7 | Biliary excretion mechanism of CPT-11 and its metabolites in humans: involvement of primary active transporters. Cancer Res. 1998 Nov 15;58(22):5137-43. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
