Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00120
|
|||||
| Drug Name |
Lapatinib
|
|||||
| Synonyms |
4-[[3-Chloro-4-(3-fluorobenzyloxy)phenyl]amino]-6-[5-[[(2-methanesulfonylethyl)amino]methyl]furan-2-yl]quinazoline; FMM; GSK 572016; GSK572016; GW 572016; GW 572016X; GW572016; Lapatinib (ERBB2 inhibitor); Lapatinib (INN); Lapatinib Ditosylate; Lapatinib [INN]; Lapatinib tosilate hydrate; Lapatinib, Tykerb, GW572016; N-(3-Chloro-4-((3-fluorophenyl)methoxy)phenyl)-6-(5-((2-methylsulfonylethylamino)methyl)-2-furyl)quinazolin-4-amine; N-(3-Chloro-4-{[(3-fluorophenyl)methyl]oxy}phenyl)-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furanyl]-4-quinazolinamine; N-[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]-6-[5-[(2-methylsulfonylethylamino)methyl]furan-2-yl]quinazolin-4-amine; N-{3-CHLORO-4-[(3-FLUOROBENZYL)OXY]PHENYL}-6-[5-({[2-(METHYLSULFONYL)ETHYL]AMINO}METHYL)-2-FURYL]-4-QUINAZOLINAMINE; Tycerb; Tykerb (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
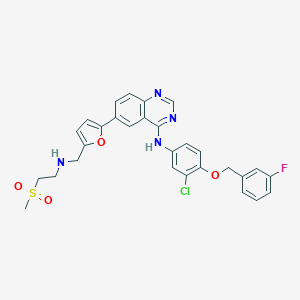
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C29H26ClFN4O4S
|
|||||
| Canonical SMILES |
CS(=O)(=O)CCNCC1=CC=C(O1)C2=CC3=C(C=C2)N=CN=C3NC4=CC(=C(C=C4)OCC5=CC(=CC=C5)F)Cl
|
|||||
| InChI |
InChI=1S/C29H26ClFN4O4S/c1-40(36,37)12-11-32-16-23-7-10-27(39-23)20-5-8-26-24(14-20)29(34-18-33-26)35-22-6-9-28(25(30)15-22)38-17-19-3-2-4-21(31)13-19/h2-10,13-15,18,32H,11-12,16-17H2,1H3,(H,33,34,35)
|
|||||
| InChIKey |
BCFGMOOMADDAQU-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 388082-78-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 581.1 | Topological Polar Surface Area | 115 | ||
| Heavy Atom Count | 40 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 9 | |||
| XLogP |
5.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103177479
, 103854383
, 103905567
, 103905568
, 109692966
, 113442073
, 117695459
, 124360113
, 124893335
, 124893336
, 125345521
, 126592984
, 126621155
, 126649062
, 126666978
, 126667073
, 126731332
, 127325943
, 127325944
, 127325945
, 127494626
, 134338132
, 135128225
, 135685383
, 135685387
, 14911387
, 21317859
, 30413551
, 46393564
, 46506302
, 46507141
, 49742619
, 50070568
, 50071307
, 50100107
, 50112760
, 50644701
, 53788364
, 57399558
, 585695
, 7887520
, 8035064
, 85171071
, 85202079
, 91147938
, 92308826
, 92719029
, 93581028
, 9368726
, 96024798
|
|||||
| ChEBI ID |
ChEBI:49603
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Lapatinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008 Apr;36(4):695-701. | |||||
| 3 | Tarascon Pocket Pharmacopoeia 2018 Classic Shirt-Pocket Edition. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
