Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00129
|
|||||
| Drug Name |
Estradiol
|
|||||
| Synonyms |
17 beta-Estradiol; 17-.BETA.-Estradiol; 17-beta-OH-estradiol; 17-beta-estradiol; 17.beta.-Estradiol; 17.beta.-Oestradiol; 17b-Oestradiol; 17beta oestradiol; 17beta-Estradiol; 17beta-Oestradiol; 3,17-beta-Estradiol; 3,17-beta-Oestradiol; 3,17.beta.-Estradiol; 3,17beta-Estradiol; Activella (TN); Aerodiol; Alora; Alora (TN); Alpha-Estradiol; Alpha-Oestradiol; Altrad; AngeliQ (TN); Aquadiol; B-Estradiol; Bardiol; Beta-Oestradiol; Beta-estradiol; CMC_11154; Cis-Estradiol; Cis-Oestradiol; Climaderm; Climara; Climara (TN); Climara Forte; Component of Menrium; Compudose; Compudose 200; Compudose 365; Corpagen; D-3,17beta-Estradiol; D-Estradiol; D-Oestradiol; Dermestril; Destradiol; Dihydrofolliculin; Dihydromenformon; Dihydrotheelin; Dihydroxyesterin; Dihydroxyestrin; Dihydroxyoestrin; Dimenformon; Diogyn; Diogynets; Divigel; Divigel (TN); E 2; E 8875; E(sub 2); E0025; Elestrin; Elestrin (TN); Encore; Epiestriol 50; Esclim; Estrace; Estrace (TN); Estraderm; Estraderm (TN); Estraderm MX; Estraderm TTS; Estraderm TTS (TN); Estraderm TTS 100; Estraderm TTS 50; Estradiol [USAN:INN]; Estradiol acetate (TN); Estradiol cypionate (TN); Estradiol valerate (TN); Estradiol-17 beta; Estradiol-17-beta; Estradiol-17beta; Estradiol-3,17beta; Estradiolo; Estradiolo [DCIT]; Estradiolum; Estradiolum [INN]; Estradot; Estraldine; Estrapak 50; Estrasorb; Estrasorb (TN); Estrasorb Topical (TN); Estreva; Estrifam; Estring; Estring (TN); Estring vaginal ring; Estroclim; Estroclim 50; Estrodiolum; Estrofem (TN); Estrofem 2; Estrofem Forte; Estrogel; Estrogel (TN); Estrovite; EvaMist (TN); Evamist; Evorel; Extrasorb; Femanest; Femestral; Femestrol; Femogen; Fempatch; Femring (TN); Femtrace; Femtran; Follicyclin; Gelestra; Ginedisc; Ginosedol; GynPolar; Gynergon; Gynestrel; Gynodiol; Gynoestryl; Innofem; Innofem (TN); Lamdiol; Macrodiol; Macrol; Menest; Menorest; Menostar (TN); Microdiol; Nordicol; Oesclim; Oestergon; Oestradiol; Oestradiol Berco; Oestradiol R; Oestradiol-17-beta; Oestradiol-17beta; Oestradiolum; Oestrogel; Oestroglandol; Oestrogynal; Ovahormon; Ovasterol; Ovastevol; Ovociclina; Ovocyclin; Ovocycline; Ovocylin; Perlatanol; Polyestradiol; Primofol; Profoliol; Profoliol B; Progynon DH; Progynon-DH; Progynon;Syndiol; Progynova (TN); S-21400; SK-Estrogens; SL-1100; Sandrena 1; Sandrena Gel; Sisare Gel; Systen; Tradelia; Trial SAT; Trocosone; VIVELLE-DOT; Vagifem; Vagifem (TN); Vivelle; Vivelle (TN); Vivelle-Dot (TN); Zerella; Zesteem; Zesteen; Zumenon; [2,4,6,7-3H]-E2; [3H]-estradiol; [3H]]estradiol
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Therapeutic Class |
Estrogens
|
|||||
| Structure |
|
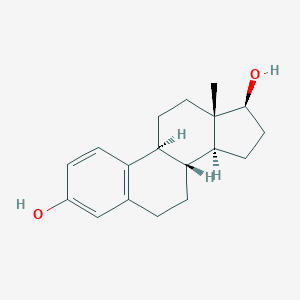 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H24O2
|
|||||
| Canonical SMILES |
CC12CCC3C(C1CCC2O)CCC4=C3C=CC(=C4)O
|
|||||
| InChI |
InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h3,5,10,14-17,19-20H,2,4,6-9H2,1H3/t14-,15-,16+,17+,18+/m1/s1
|
|||||
| InChIKey |
VOXZDWNPVJITMN-ZBRFXRBCSA-N
|
|||||
| CAS Number |
CAS 50-28-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 272.4 | Topological Polar Surface Area | 40.5 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 0 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 2 | |||
| XLogP |
4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321307
, 11110639
, 11120283
, 11120771
, 11121259
, 11121708
, 11122188
, 11362819
, 11364683
, 11365381
, 11367245
, 11367943
, 11369807
, 11370869
, 11370870
, 11372848
, 11373544
, 11375407
, 11376105
, 11377970
, 11466469
, 11467589
, 11486157
, 12012600
, 14799365
, 14799367
, 17389898
, 17405066
, 2384
, 4202
, 4266382
, 652955
, 75249
, 7847173
, 7887363
, 8028360
, 8139856
, 8139865
, 8143549
, 8153534
, 820385
, 821147
, 823461
, 823615
, 825708
, 829421
, 831023
, 831024
, 838509
, 841780
|
|||||
| ChEBI ID |
ChEBI:16469
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Estradiol was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Sterol transport by the human breast cancer resistance protein (ABCG2) expressed in Lactococcus lactis. J Biol Chem. 2003 Jun 6;278(23):20645-51. | |||||
| 3 | Antiestrogens and steroid hormones: substrates of the human P-glycoprotein. Biochem Pharmacol. 1994 Jul 19;48(2):287-92. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
