Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00158
|
|||||
| Drug Name |
Glimepiride
|
|||||
| Synonyms |
1-((p-(2-(3-Ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl)phenyl)sulfonyl)-3-(trans-4-methylcyclohexyl)urea; 1-{[4-(2-{[(3-ethyl-4-methyl-2-oxo-2,5-dihydro-1H-pyrrol-1-yl)carbonyl]amino}ethyl)phenyl]sulfonyl}-3-(trans-4-methylcyclohexyl)urea; 3-ethyl-4-methyl-N-[2-(4-{[(trans-4-methylcyclohexyl)carbamoyl]sulfamoyl}phenyl)ethyl]-2-oxo-2,5-dihydro-1H-pyrrole-1-carboxamide; 3-ethyl-4-methyl-n-(4-(n-((1r,4r)-4-methylcyclohexylcarbamoyl)sulfamoyl)phenethyl)-2-oxo-2,5-dihydro; 4-ethyl-3-methyl-N-[2-[4-[(4-methylcyclohexyl)carbamoylsulfamoyl]phenyl]ethyl]-5-oxo-2H-pyrrole-1-carboxamide; 64598P; Amarel; Amaryl; Amaryl (TN); Amaryl, Glista OD, Glimepiride; Endial; Glimepirid; Glimepirida; Glimepirida [Spanish]; Glimepiride (JAN/USP/INN); Glimepiride [USAN:BAN:INN]; Glimepiridum; Glimepiridum [Latin]; Glimepride; Glimer; Glymepirid; HOE 490; Hoe-490; Novo-glimepiride; PMS-glimepiride; Ratio-glimepiride; Roname; Sandoz glimepiride; Solosa
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Diabetes [ICD11: 5A10-5A14] | Approved | [1] | |||
| Therapeutic Class |
Hypoglycemic Agents
|
|||||
| Structure |
|
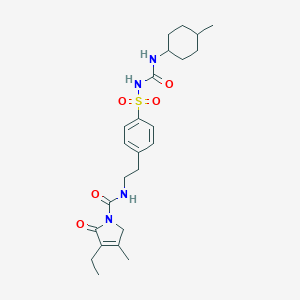 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H34N4O5S
|
|||||
| Canonical SMILES |
CCC1=C(CN(C1=O)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCC(CC3)C)C
|
|||||
| InChI |
InChI=1S/C24H34N4O5S/c1-4-21-17(3)15-28(22(21)29)24(31)25-14-13-18-7-11-20(12-8-18)34(32,33)27-23(30)26-19-9-5-16(2)6-10-19/h7-8,11-12,16,19H,4-6,9-10,13-15H2,1-3H3,(H,25,31)(H2,26,27,30)
|
|||||
| InChIKey |
WIGIZIANZCJQQY-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 93479-97-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 490.6 | Topological Polar Surface Area | 133 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
3.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103394534
, 103543151
, 103913636
, 104171333
, 104303626
, 11112876
, 11466679
, 11467799
, 11486338
, 12013623
, 14908413
, 24895093
, 26719876
, 26755029
, 26755030
, 29222609
, 32963623
, 46386690
, 46508842
, 47589079
, 47959862
, 48155569
, 48185080
, 48334594
, 49648856
, 49699123
, 49731995
, 49835734
, 50037704
, 50126308
, 5043708
, 56313637
, 57321825
, 77374551
, 7847659
, 7979409
, 81040871
, 81092812
, 8145852
, 8152210
, 85787655
, 85787837
, 91613422
, 92125647
, 92308643
, 92309214
, 92711714
, 9871
, 99344865
, 99437102
|
|||||
| ChEBI ID |
ChEBI:5383
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BSEP | Transporter Info | Bile salt export pump | Substrate | [2] | |
| References | ||||||
| 1 | Glimepiride was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
