Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00162
|
|||||
| Drug Name |
Etoposide
|
|||||
| Synonyms |
(-)-Etoposide; 4'-Demethyl-epipodophyllotoxin 9-[4,6-O-(R)-ethylidene-beta-D-glucopyranoside; 4'-Demethylepipodophyllotoxin 9-(4,6-O-(R)-ethylidene-beta-D-glucopyranoside); 4'-Demethylepipodophyllotoxin 9-(4,6-O-ethylidene-beta-D-glucopyranoside); 4'-Demethylepipodophyllotoxin ethylidene-.beta.-D-glucoside; 4'-O-Demethyl-1-O-(4,6-O-ethylidene-beta-D-glucopyranosyl)epipodophyllotoxin; 4-Demethylepipodophyllotoxin beta-D-ethylideneglucoside; 4-Demethylepipodophyllotoxin-.beta.-D-ethylideneglucoside; DEMETHY-EPIPODOPHYLLOTOXIN, ETHYLIDENE GLUCOSIDE; Demethyl EpipodophyllotoxinEthylidine Glucoside; Demethyl-epiodophyllotoxin ethylidene glucoside; Demethylepipodophyllotoxin-beta-D-ethylideneglucoside; E0675; Epipodophyllotoxin VP-16213; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-.beta.-D-glucopyranoside; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside; Epipodophyllotoxin, 4'-demethyl-, 4,6-O-ethylidene-beta-D-glucopyranoside (8CI); Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-.beta.-D-glucopyranoside); Epipodophyllotoxin, 4'-demethyl-, 9-(4,6-O-ethylidene-beta-D-glucopyranoside); Eposide; Eposin; Eposin (TN); Eposin, Vepesid, VP-16, Toposar, Etoposide; Etopol; Etopophos (TN); Etopophos (phosphate salt);Etoposide (VP16); Etoposide (JP15/USP/INN); Etoposide [USAN:INN:BAN:JAN]; Etoposido; Etoposido [INN-Spanish]; Etoposidum; Etoposidum [INN-Latin]; Etosid; Lastet; NK 171; Toposar; Trans-Etoposide; VP 16; VP 16 (pharmaceutical); VP 16-213; VP 16213; VP-16; VP-16 (TN); VP-16-213; VePESID (TN); VePesid; Vepesid J; Vepeside; Zuyeyidal
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Testicular cancer [ICD11: 2C80] | Approved | [1] | |||
| Kaposi's sarcoma [ICD11: 2B57] | Approved | [1] | ||||
| Ewing's sarcoma [ICD11: 2B52] | Approved | [1] | ||||
| Lung cancer [ICD11: 2C25] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
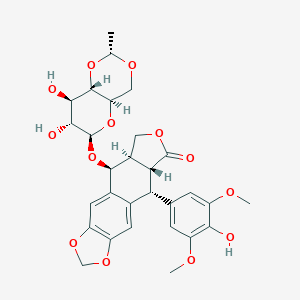 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C29H32O13
|
|||||
| Canonical SMILES |
CC1OCC2C(O1)C(C(C(O2)OC3C4COC(=O)C4C(C5=CC6=C(C=C35)OCO6)C7=CC(=C(C(=C7)OC)O)OC)O)O
|
|||||
| InChI |
InChI=1S/C29H32O13/c1-11-36-9-20-27(40-11)24(31)25(32)29(41-20)42-26-14-7-17-16(38-10-39-17)6-13(14)21(22-15(26)8-37-28(22)33)12-4-18(34-2)23(30)19(5-12)35-3/h4-7,11,15,20-22,24-27,29-32H,8-10H2,1-3H3/t11-,15+,20-,21-,22+,24-,25-,26-,27-,29+/m1/s1
|
|||||
| InChIKey |
VJJPUSNTGOMMGY-MRVIYFEKSA-N
|
|||||
| CAS Number |
CAS 33419-42-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 588.6 | Topological Polar Surface Area | 161 | ||
| Heavy Atom Count | 42 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 13 | |||
| XLogP |
0.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
104019076
, 104234176
, 104324084
, 11110521
, 117664408
, 117682510
, 119525100
, 12013288
, 121363248
, 124349593
, 124659141
, 124757097
, 124800231
, 124893613
, 125163901
, 127298729
, 127298730
, 127298731
, 127298732
, 14886949
, 14935915
, 24278178
, 24769897
, 34677904
, 46386954
, 46500626
, 46505434
, 4733
, 47362874
, 48182835
, 48332078
, 49681767
, 49699016
, 49855364
, 53787668
, 56463308
, 57311878
, 597749
, 71825014
, 75974183
, 7847193
, 7979208
, 8145856
, 8174504
, 85148351
, 85788790
, 89850276
, 92308660
, 92308790
, 99437037
|
|||||
| ChEBI ID |
ChEBI:4911
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [5] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [6] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [7] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [8] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP2 | Transporter Info | Km = 617 microM | Madin-Darby canine kidney cells (MDCKII)-MRP2 | [4] | |
| MRP3 | Transporter Info | Km = 11.4 microM | Spodoptera frugiperda (Sf9) cells-MRP3 | [9] | ||
| P-GP | Transporter Info | Km = 461 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [8] | ||
| P-GP | Transporter Info | Km = 255 microM | Madin-Darby canine kidney cells (MDCKII)-MDR1 | [4] | ||
| References | ||||||
| 1 | Etoposide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Characterization of 3-methoxy flavones for their interaction with ABCG2 as suggested by ATPase activity. Biochim Biophys Acta. 2014 Nov;1838(11):2929-38. | |||||
| 3 | Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J Pharmacol Exp Ther. 2001 Sep;298(3):1199-205. | |||||
| 4 | Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT). Drug Metab Dispos. 2002 Apr;30(4):457-63. | |||||
| 5 | Functional reconstitution of human ABCC3 into proteoliposomes reveals a transport mechanism with positive cooperativity. Biochemistry. 2009 May 26;48(20):4423-30. | |||||
| 6 | Identification of drugs and drug metabolites as substrates of multidrug resistance protein 2 (MRP2) using triple-transfected MDCK-OATP1B1-UGT1A1-MRP2 cells. Br J Pharmacol. 2012 Mar;165(6):1836-1847. | |||||
| 7 | Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008 Apr 14;584(1):57-65. | |||||
| 8 | Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm Res. 2003 Aug;20(8):1200-9. | |||||
| 9 | Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3). J Biol Chem. 2001 Dec 7;276(49):46400-7. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
