Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00164
|
|||||
| Drug Name |
Dabrafenib
|
|||||
| Synonyms |
1195765-45-7; CHEBI:75045; Dabrafenib (GSK2118436); Dabrafenib [USAN:INN]; GSK 2118436; GSK-2118436A; GSK2118436; GSK2118436A; N-(3-(5-(2-aminopyrimidin-4-yl)-2-tert-butylthiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenesulfonamide; N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide; N-{3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide; QGP4HA4G1B; Tafinlar; UNII-QGP4HA4G1B
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Melanoma [ICD11: 2C30] | Approved | [1] | |||
| Structure |
|
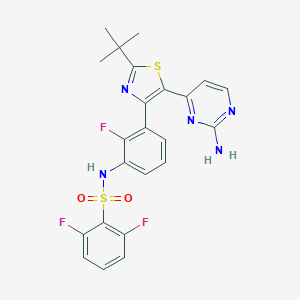 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C23H20F3N5O2S2
|
|||||
| Canonical SMILES |
CC(C)(C)C1=NC(=C(S1)C2=NC(=NC=C2)N)C3=C(C(=CC=C3)NS(=O)(=O)C4=C(C=CC=C4F)F)F
|
|||||
| InChI |
InChI=1S/C23H20F3N5O2S2/c1-23(2,3)21-30-18(19(34-21)16-10-11-28-22(27)29-16)12-6-4-9-15(17(12)26)31-35(32,33)20-13(24)7-5-8-14(20)25/h4-11,31H,1-3H3,(H2,27,28,29)
|
|||||
| InChIKey |
BFSMGDJOXZAERB-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 1195765-45-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 519.6 | Topological Polar Surface Area | 148 | ||
| Heavy Atom Count | 35 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 11 | |||
| XLogP |
4.8
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
ChEBI:75045
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Dabrafenib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mechanisms limiting distribution of the threonine-protein kinase B-RaF(V600E) inhibitor dabrafenib to the brain: implications for the treatment of melanoma brain metastases. J Pharmacol Exp Ther. 2013 Mar;344(3):655-64. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
