Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00170
|
|||||
| Drug Name |
Epirubicin
|
|||||
| Synonyms |
(1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranoside; (1S,3S)-3,5,12-trihydroxy-3-(hydroxyacetyl)-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-arabino-hexopyranoside; (7S,9R)-7-[(2S,4S,5R,6S)-4-Amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-7-[(2R,4S,5R,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; 10-((3-amino-2,3,6-trideoxy-beta-L-arabino-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxyacetyl)-1-methoxy-(8S-cis)-5,12-naphthacenedione; 4'-Epi-DXR; 4'-Epiadriamycin; 4'-epi-DX; 4'-epi-Doxorubicin; 4'-epidoxorubicin; 4-Epidoxorubicin; Ebewe (TN); Ellence; Ellence (TN); Epi-DX; Epiadriamycin; Epidoxorubicin; Epirubicin (INN); Epirubicin (TN); Epirubicin [INN:BAN]; Epirubicina; Epirubicina [INN-Spanish]; Epirubicina [Spanish]; Epirubicine; Epirubicine [French]; Epirubicine [INN-French]; Epirubicinum; Epirubicinum [INN-Latin]; Epirubicinum [Latin]; Farmorubicin (TN); IMI 28; Pharmorubicin (TN); Pharmorubicin Pfs; Pidorubicin; Pidorubicina; Pidorubicina [INN-Spanish]; Pidorubicine; Pidorubicine [INN-French]; Pidorubicinum; Pidorubicinum [INN-Latin]; Ridorubicin; WP 697
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Node-positive breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
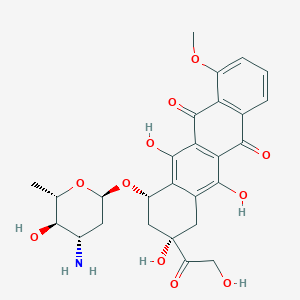 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C27H29NO11
|
|||||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=C(C4=O)C(=CC=C5)OC)O)(C(=O)CO)O)N)O
|
|||||
| InChI |
InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22-,27-/m0/s1
|
|||||
| InChIKey |
AOJJSUZBOXZQNB-VTZDEGQISA-N
|
|||||
| CAS Number |
CAS 56420-45-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 543.5 | Topological Polar Surface Area | 206 | ||
| Heavy Atom Count | 39 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 6 | Hydrogen Bond Acceptor Count | 12 | |||
| XLogP |
1.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103164726
, 104338146
, 117597720
, 123080197
, 126686290
, 127301368
, 127301369
, 127301370
, 127301371
, 127301372
, 127301373
, 127301374
, 127301375
, 127301376
, 127301377
, 127301378
, 127301379
, 127301380
, 127301381
, 127301382
, 127301383
, 127301384
, 127301385
, 127301386
, 127301387
, 127301388
, 13409
, 14716206
, 14812446
, 14837077
, 24769893
, 26704252
, 26710264
, 34707467
, 46507282
, 46530809
, 48415946
, 49995002
, 50070726
, 53787927
, 56311421
, 56313206
, 56313988
, 57288587
, 57288773
, 57312617
, 77126435
, 793944
, 8177324
, 96024597
|
|||||
| ChEBI ID |
ChEBI:47898
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MRP1 | Transporter Info | Km = 0.097 microM | Human cervical cancer cell line (Hela)-MRP1 | [3] | |
| References | ||||||
| 1 | Epirubicin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | Sulindac sulfide selectively increases sensitivity of ABCC1 expressing tumor cells to doxorubicin and glutathione depletion. J Biomed Res. 2016 Mar;30(2):120-133. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
