Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00176
|
|||||
| Drug Name |
Ribavirin
|
|||||
| Synonyms |
1-.beta.-D-Ribofuranosyl-1,2,4-triazolo-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,2,4-triazole-3-carboxamide; 1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-1,2,4-triazole-3-carboxamide; 1-beta-D-Ribofuranosyl-1,2,4-triazole-3-carboxamide; 1-beta-D-Ribofuranosyl-1H-1,2,4-triazole-3-carboxamide; 1-beta-D-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide; AA-504/07617051; C-Virin; Copegus; Copegus (TN); Cotronak; DRG-0028; Drug: Ribavirin; ICN-1229; KS-1104; R 9644; R-964; RBV; RG-964; RTC; RTCA; Ravanex; Rebetol; Rebetol (TN); Rebetron; Rebretron; Ribacine; Ribamide; Ribamidil; Ribamidyl; Ribasphere; Ribasphere (TN); Ribav; Ribavirin (JAN/USP/INN); Ribavirin Capsules; Ribavirin [USAN:INN]; Ribavirina; Ribavirina [INN-Spanish]; Ribavirine; Ribavirine [INN-French]; Ribavirinum; Ribavirinum [INN-Latin]; Ribovirin; Ro 20-9963/000; Ro-20-9963; SCH 18908; Tribavirin; Varazid; Vilona; Vilona (TN); Viramid; Viramide; Virazid; Virazide; Virazole; Virazole (Ribavirin) Inhalation Solution; Virazole (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hepatitis C virus infection [ICD11: 1E50.2, 1E51.1] | Approved | [1] | |||
| Therapeutic Class |
Antiviral Agents
|
|||||
| Structure |
|
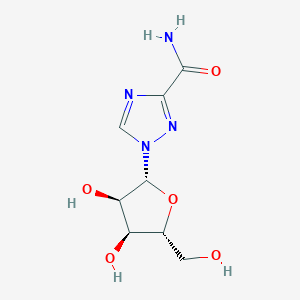 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H12N4O5
|
|||||
| Canonical SMILES |
C1=NC(=NN1C2C(C(C(O2)CO)O)O)C(=O)N
|
|||||
| InChI |
InChI=1S/C8H12N4O5/c9-6(16)7-10-2-12(11-7)8-5(15)4(14)3(1-13)17-8/h2-5,8,13-15H,1H2,(H2,9,16)/t3-,4-,5-,8-/m1/s1
|
|||||
| InChIKey |
IWUCXVSUMQZMFG-AFCXAGJDSA-N
|
|||||
| CAS Number |
CAS 36791-04-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 244.2 | Topological Polar Surface Area | 144 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-1.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11335957
, 11361196
, 11364740
, 11367302
, 11369864
, 11372951
, 11374942
, 11378029
, 11462168
, 11491691
, 11493066
, 11495640
, 11528313
, 12059582
, 14798456
, 15122005
, 17389529
, 17405619
, 24278685
, 25621753
, 26538475
, 26612558
, 26679740
, 34678832
, 46505883
, 47291173
, 47365228
, 47440296
, 47515344
, 47589035
, 47885447
, 47885448
, 48035157
, 48416516
, 48424047
, 48425596
, 48631153
, 49699015
, 50105410
, 50105411
, 50105412
, 50105413
, 50474786
, 53778169
, 595938
, 7847489
, 7980513
, 8139972
, 8150084
, 8175073
|
|||||
| ChEBI ID |
CHEBI:63580
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | CNT2 | Transporter Info | Concentrative nucleoside transporter 2 | Substrate | [2] | |
| CNT3 | Transporter Info | Concentrative Na(+)-nucleoside cotransporter 3 | Substrate | [3] | ||
| ENT1 | Transporter Info | Equilibrative nucleoside transporter 1 | Substrate | [4] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | CNT3 | Transporter Info | Km = 28.2 microM | Xenopus oocytes-hCNT3 and hENT1 | [3] | |
| References | ||||||
| 1 | Ribavirin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification and functional analysis of variants in the human concentrative nucleoside transporter 2, hCNT2 (SLC28A2) in Chinese, Malays and Indians. Pharmacogenet Genomics. 2007 Sep;17(9):783-6. | |||||
| 3 | Kinetic study of anti-viral ribavirin uptake mediated by hCNT3 and hENT1 in Xenopus laevis oocytes. Biophys Chem. 2010 Mar;147(1-2):59-65. | |||||
| 4 | Effects of dipyridamole coadministration on the pharmacokinetics of ribavirin in healthy volunteers. Drug Metab Pharmacokinet. 2013;28(5):406-10. | |||||
| 5 | Transport of levovirin prodrugs in the human intestinal Caco-2 cell line. J Pharm Sci. 2006 Jun;95(6):1318-25. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
