Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00182
|
|||||
| Drug Name |
Dasatinib
|
|||||
| Synonyms |
(18F)-N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide; 2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)-N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide; BMS 354825; BMS-354825; BMS-354825, Sprycel, BMS354825, Dasatinib; BMS354825; Dasatinib (USAN); Dasatinib [USAN]; Dasatinib anhydrous; Dasatinib, BMS 354825; Dasatinibum; N-(2-CHLORO-6-METHYLPHENYL)-2-({6-[4-(2-HYDROXYETHYL)PIPERAZIN-1-YL]-2-METHYLPYRIMIDIN-4-YL}AMINO)-1,3-THIAZOLE-5-CARBOXAMIDE; N-(2-Chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazolecarboxamide, monohydrate; N-(2-chloro-6-methylphenyl)-2-((6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-yl)amino)thiazole-5-carboxamide; N-(2-chloro-6-methylphenyl)-2-(6-(4-(2-hydroxyethyl)piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide; N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)piperazin-1-yl]-2-methylpyrimidin-4-yl]amino]-1,3-thiazole-5-carboxamide; SPRYCEL (TN); Sprycel; Spyrcel
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Multiple myeloma [ICD11: 2A83] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
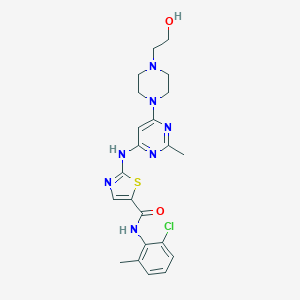 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H26ClN7O2S
|
|||||
| Canonical SMILES |
CC1=C(C(=CC=C1)Cl)NC(=O)C2=CN=C(S2)NC3=CC(=NC(=N3)C)N4CCN(CC4)CCO
|
|||||
| InChI |
InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27)
|
|||||
| InChIKey |
ZBNZXTGUTAYRHI-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 302962-49-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 488 | Topological Polar Surface Area | 135 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 9 | |||
| XLogP |
3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10061134
, 103501621
, 103905543
, 111644212
, 117418208
, 117695728
, 118050493
, 119503515
, 12015788
, 124361516
, 124756940
, 124893650
, 124893651
, 124893652
, 125001912
, 125163747
, 125264620
, 125347447
, 126624063
, 14859389
, 17397755
, 17422520
, 36127155
, 46505143
, 46513626
, 47883136
, 48429642
, 49742622
, 50070229
, 50070567
, 50071309
, 50100097
, 50125840
, 52995488
, 53799228
, 56312240
, 56312839
, 56313127
, 56373968
, 56459420
, 57288451
, 57354110
, 57551950
, 74374210
, 85246174
, 87235888
, 87357382
, 93581026
, 99381604
, 99437986
|
|||||
| ChEBI ID |
ChEBI:49375
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Dasatinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009 Oct;158(4):1153-64. | |||||
| 3 | Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013 Mar 15;19(6):1458-66. | |||||
| 4 | Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010 Aug;38(8):1371-80. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
