Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00201
|
|||||
| Drug Name |
Sulfasalazine
|
|||||
| Synonyms |
13gs; 2-HYDROXY-(5-([4-(2-PYRIDINYLAMINO)SULFONYL]PHENYL)AZO)BENZOIC ACID; 2-Hydroxy-5-((4-((2-pyridinylamino)sulfonyl)phenyl)azo)benzoic acid; 2-hydroxy-5-(4-[(pyridin-2-ylamino)sulfonyl]phenyl}diazenyl)benzoic acid; 2-hydroxy-5-[(E)-{4-[(pyridin-2-ylamino)sulfonyl]phenyl}diazenyl]benzoic acid; 2-hydroxy-5-{[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl}benzoic acid; 4-(Pyridyl-2-amidosulfonyl)-3'-carboxy-4'-hydroxyazobenzene; 5-((p-(2-Pyridylsulfamoyl)phenyl)azo)salicylic acid; 5-(4-(2-Pyridylsulfamoyl)phenylazo)-2-hydroxybenzoic acid; 5-(p-(2-Pyridinylsulfamoyl)Phenylazo)Salicylic Acid; 5-(p-(2-Pyridylsulfamyl)phenylazo)salicylic acid; 5-(para-(2-pyridylsulfamoyl)phenylazo)salicylic acid; 5-[4-(2-Pyridylsulfamoyl)-phenylazo]-salicylic acid; 5-[4-(2-Pyridylsulfamoyl)phenylazo]salicylic Acid; 5-[p-(2-Pyridylsulfamoyl)phenylazo]salicylic acid; 5-[p-(2-Pyridylsulfamyl)phenylazo]salicylic acid; Accucol; Alphapharm Brand of Sulfasalazine; Alti-Sulfasalazine; Ashbourne Brand of Sulfasalazine; Asulfidine; Azlufidine EN-Tabs; Azopyrin; Azopyrine; Azosulfidin; Azulfadine; Azulfidine; Azulfidine (TN); Azulfidine EN; Azulfidine EN-tabs; Benzosulfa; Colo Pleon; Colo-Pleon; EN, Azulfidine; FNA Brand of Sulfasalazine; Henning Berlin Brand of Sulfasalazine; Heyl Brand of Sulfasalazine; Medac Brand of Sulfasalazine; PMS-Sulfasalazine; Pfizer Brand of Sulfasalazine; Pleon; Pms-Sulfasalazine E.C.; Pyralin EN; Ratio Sulfasalazine; Ratio-Sulfasalazine; Ratiopharm Brandof Sulfasalazine; Reupirin; S.A.S. 500; S.A.S. Enteric-500; S.A.S.-500; SASP; SI-88; SSZ; Salazo-sulfapyridinum; Salazopiridazin; Salazopyridin; Salazopyrin; Salazopyrin (TN); Salazopyrin EN-Tabs; Salazosulfapiridina; Salazosulfapiridina [INN-Spanish]; Salazosulfapyridin; Salazosulfapyridine; Salazosulfapyridine (JP15); Salazosulfapyridinum; Salazosulfapyridinum [INN-Latin]; Salicylazosulfapyridine; Salisulf; Sanofi Synthelabo Brand of Sulfasalazine; Sas-500; Sulcolon; Sulfasalazin; Sulfasalazin Heyl; Sulfasalazin medac; Sulfasalazin-Heyl; Sulfasalazina; Sulfasalazina [INN-Spanish]; Sulfasalazine (USP/INN); Sulfasalazine FNA; Sulfasalazine Pfizer Brand; Sulfasalazine [USAN:INN]; Sulfasalazinum; Sulfasalazinum [INN-Latin]; Sulfazalazine; Sulphasalazine; Ucine; Ulcol; W-t Sasp oral
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Inflammatory bowel disease [ICD11: DD7Z] | Approved | [1] | |||
| Rheumatoid arthritis [ICD11: FA20] | Approved | [1] | ||||
| Therapeutic Class |
Antiinflammatory Agents
|
|||||
| Structure |
|
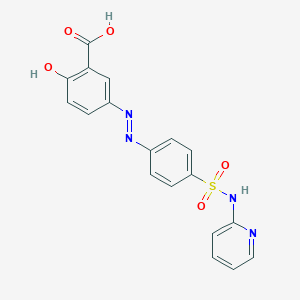 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H14N4O5S
|
|||||
| Canonical SMILES |
C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N=NC3=CC(=C(C=C3)O)C(=O)O
|
|||||
| InChI |
InChI=1S/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25)
|
|||||
| InChIKey |
NCEXYHBECQHGNR-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 599-79-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 398.4 | Topological Polar Surface Area | 150 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 9 | |||
| XLogP |
-0.7
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
ChEBI:9334
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [4] | ||
| PEPT1 | Transporter Info | Peptide transporter 1 | Substrate | [5] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | BCRP | Transporter Info | Km = 0.7 microM | Spodoptera frugiperda (Sf9) cells-BCRP | [6] | |
| References | ||||||
| 1 | Sulfasalazine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm Res. 2009 Feb;26(2):480-7. | |||||
| 3 | Small intestinal efflux mediated by MRP2 and BCRP shifts sulfasalazine intestinal permeability from high to low, enabling its colonic targeting. Am J Physiol Gastrointest Liver Physiol. 2009 Aug;297(2):G371-7. | |||||
| 4 | Small-Dosing Clinical Study: Pharmacokinetic, Pharmacogenomic (SLCO2B1 and ABCG2), and Interaction (Atorvastatin and Grapefruit Juice) Profiles of 5 Probes for OATP2B1 and BCRP. J Pharm Sci. 2017 Sep;106(9):2688-2694. | |||||
| 5 | Peptide transporter substrate identification during permeability screening in drug discovery: comparison of transfected MDCK-hPepT1 cells to Caco-2 cells. Arch Pharm Res. 2007 Apr;30(4):507-18. | |||||
| 6 | Kinetic characterization of sulfasalazine transport by human ATP-binding cassette G2. Biol Pharm Bull. 2009 Mar;32(3):497-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
