Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00204
|
|||||
| Drug Name |
Ponatinib
|
|||||
| Synonyms |
Iclusig (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acute lymphoblastic leukemia [ICD11: 2B33.0] | Approved | [1] | |||
| Structure |
|
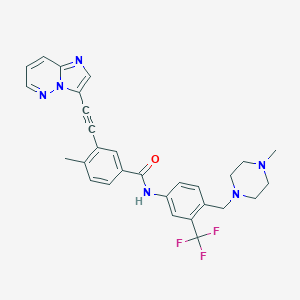 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C29H27F3N6O
|
|||||
| Canonical SMILES |
CC1=C(C=C(C=C1)C(=O)NC2=CC(=C(C=C2)CN3CCN(CC3)C)C(F)(F)F)C#CC4=CN=C5N4N=CC=C5
|
|||||
| InChI |
InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39)
|
|||||
| InChIKey |
PHXJVRSECIGDHY-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 943319-70-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 532.6 | Topological Polar Surface Area | 65.8 | ||
| Heavy Atom Count | 39 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
4.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103162345
, 103768137
, 104144064
, 124757292
, 125164096
, 131480900
, 135263267
, 135626676
, 135727465
, 136350016
, 136367447
, 136368095
, 136920265
, 137245313
, 137275996
, 139832791
, 144115614
, 152134633
, 152146116
, 152258369
, 160644559
, 160647206
, 162011539
, 162037632
, 162164010
, 162164012
, 162201514
, 163209942
, 164043013
, 164187223
, 164834148
, 170503740
, 172087035
, 174006583
, 174531620
, 175266091
, 175427142
, 177749778
, 178102513
, 180371706
, 185988972
, 188899573
, 196408591
, 198993325
, 204380154
, 49848164
, 57136216
, 85267726
, 85267727
, 99437188
|
|||||
| ChEBI ID |
ChEBI:78543
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Ponatinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018 Jan 4;46(D1):D1074-D1082. (ID: DB08901) | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
