Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00205
|
|||||
| Drug Name |
Riluzole
|
|||||
| Synonyms |
2-Amino-6-(trifluoromethoxy)-benzothiazole; 2-Amino-6-(trifluoromethoxy)benzothiazole; 2-Amino-6-trifluoro-methoxybenzothiazole; 2-amino-6-(trifluoromethoxy)-1,3-benzothiazole; 2-amino-6-(trifluoromethoxy)benzo[d]thiazole; 2-amino-6-(trifluoromethoxyl)benzothiazole; 2-amino-6-trifluoromethoxybenzothiazole; 6-(trifluoromethoxy)-1,3-benzothiazol-2-amine; 6-(trifluoromethoxy)benzo[d]thiazol-2-amine; 6-Trifluoromethoxy-benzothiazol-2-ylamine; 6-trifluoromethoxybenzothiazole-2-yl-amine; ALBB-006046; Amino-2 trifluoromethoxy-6 benzothiazole; Amino-2 trifluoromethoxy-6 benzothiazole [French]; BF-37; PK-26124; PK-26124, RP-54274, Rilutek, Riluzole; R-116; RP 54274; RP-54274; Rilutek; Rilutek (TN); Riluzol; Riluzol [INN-Spanish]; Riluzole (JAN/USAN/INN); Riluzole HCl; Riluzole [USAN:INN]; Riluzolum; Riluzolum [INN-Latin]; ZERO/001785
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Amyotrophic lateral sclerosis [ICD11: 8B60.0] | Approved | [1] | |||
| Therapeutic Class |
Anticonvulsants
|
|||||
| Structure |
|
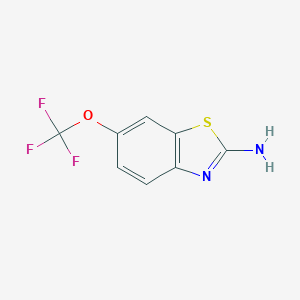 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C8H5F3N2OS
|
|||||
| Canonical SMILES |
C1=CC2=C(C=C1OC(F)(F)F)SC(=N2)N
|
|||||
| InChI |
InChI=1S/C8H5F3N2OS/c9-8(10,11)14-4-1-2-5-6(3-4)15-7(12)13-5/h1-3H,(H2,12,13)
|
|||||
| InChIKey |
FTALBRSUTCGOEG-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 1744-22-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 234.2 | Topological Polar Surface Area | 76.4 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10139
, 11111722
, 11111723
, 11113346
, 11120296
, 11120784
, 11121272
, 11466195
, 11467315
, 11484335
, 11485690
, 11488366
, 12013649
, 14773880
, 17405620
, 24278006
, 26679689
, 26751529
, 29224139
, 3158794
, 46508094
, 47440015
, 47515108
, 47588789
, 47810535
, 47810536
, 48034873
, 48334245
, 49698375
, 49890627
, 50100339
, 50104104
, 50104105
, 53778170
, 57322593
, 57566695
, 57641351
, 7379879
, 74971764
, 7735262
, 7847840
, 7980518
, 8153119
, 85089205
, 85171908
, 85231207
, 855844
, 85787918
, 85788320
, 866566
|
|||||
| ChEBI ID |
CHEBI:8863
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| References | ||||||
| 1 | Riluzole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Interactions between riluzole and ABCG2/BCRP transporter. Neurosci Lett. 2009 Mar 6;452(1):12-6. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
