Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00207
|
|||||
| Drug Name |
Amiloride
|
|||||
| Synonyms |
3,5-Diamino-N-(aminoiminomethyl)-6-chloropyrazinecarboxamide; 3,5-diamino-6-chloro-N-(diaminomethylidene)pyrazine-2-carboxamide; 3,5-diamino-N-[amino(imino)methyl]-6-chloropyrazine-2-carboxamide; 3,5-diamino-N-carbamimidoyl-6-chloropyrazine-2-carboxamide; AMILORIDE (SEE ALSO: AMILORIDE HCL (2016-88-8)); Amiclaran; Amiclaran (TN); Amikal (Hydrochloride dihydrate); Amilorida; Amilorida [INN-Spanish]; Amiloride (INN); Amiloride (Na-Ca chanel blocker); Amiloride HCL; Amiloride [INN:BAN]; Amiloride hydrochloride hydrate; Amiloridum; Amiloridum [INN-Latin]; Amipramidin; Amipramizid; Amipramizide; Amiprazidine; Amyloride; Biduret (TN); Guanamprazin; Guanamprazine; MK-870 (Hydrochloride dihydrate); Midamor; Midamor (Hydrochloride dihydrate); N-Amidino-3,5-diamino-6-chloropyrazinecarboxamide; N-Amidino-3,5-diamino-6-chlorpyrazincarboxamid; Pyrazinecarboxamide, 3,5-diamino-N-(aminoiminomethyl)-6-chloro-, monohydrochloride
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Congestive heart failure [ICD11: BD10] | Approved | [1] | |||
| High blood pressure [ICD11: BA00] | Approved | [1] | ||||
| Therapeutic Class |
Diuretics
|
|||||
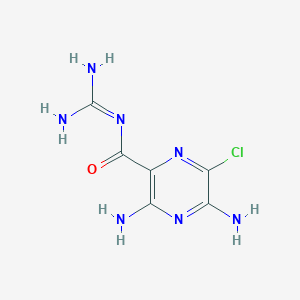
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C6H8ClN7O
|
|||||
| Canonical SMILES |
C1(=C(N=C(C(=N1)Cl)N)N)C(=O)N=C(N)N
|
|||||
| InChI |
InChI=1S/C6H8ClN7O/c7-2-4(9)13-3(8)1(12-2)5(15)14-6(10)11/h(H4,8,9,13)(H4,10,11,14,15)
|
|||||
| InChIKey |
XSDQTOBWRPYKKA-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 2016-88-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 229.63 | Topological Polar Surface Area | 159 | ||
| Heavy Atom Count | 15 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
-0.7
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11110775
, 11113372
, 11120240
, 11120728
, 11121216
, 11121637
, 11122117
, 11335357
, 11360596
, 11362706
, 11362808
, 11365268
, 11365370
, 11367830
, 11367932
, 11370683
, 11370684
, 11371426
, 11373431
, 11373683
, 11375992
, 11376094
, 11461568
, 11466035
, 11467155
, 11483872
, 11485670
, 11487934
, 11490109
, 11491947
, 11493848
, 14798141
, 26751628
, 26751629
, 29284103
, 3182570
, 46391861
, 46508156
, 46516430
, 47217018
, 47217019
, 47291344
, 47440507
, 47440508
, 5866713
, 621650
, 7885870
, 8162159
, 838821
, 9039
|
|||||
| ChEBI ID |
ChEBI:2639
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| OCT-2 | Transporter Info | Organic cation transporter 2 | Substrate | [3] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | OCT-2 | Transporter Info | Km = 95 microM | Human embryonic kidney cells (HEK293)-OCT2 | [3] | |
| References | ||||||
| 1 | Amiloride was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 3 | Characterization of regulatory mechanisms and states of human organic cation transporter 2. Am J Physiol Cell Physiol. 2006 Jun;290(6):C1521-31. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
