Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00217
|
|||||
| Drug Name |
Gabapentin
|
|||||
| Synonyms |
1-(Aminomethyl)-cyclohexaneacetic acid; 1-(Aminomethyl)cyclohexaneacetic acid; 2-[1-(aminomethyl)cyclohexyl]acetic acid; Aclonium; Apo-Gabapentin; Apotex brand of gabapentin; Aventis brand of gabapentin; DDS-2003; DM-1796; DM-5689; G-154; GBN; GOE 2450; Gabapen; Gabapentin (JAN/USAN/INN); Gabapentin (Neurontin); Gabapentin GR; Gabapentin Hexal; Gabapentin Stada; Gabapentin [USAN:INN:BAN]; Gabapentin-ratiopharm; Gabapentina; Gabapentine; Gabapentine [INN-French]; Gabapentinium; Gabapentino; Gabapentino [INN-Spanish]; Gabapentino [Spanish]; Gabapentinum; Gabapentinum [INN-Latin]; Gabapetin; Go 3450; Goe-3450; Hexal brand of gabapentin; Neurontin; Neurontin (TN); Novo-Gabapentin; Novopharm brand of gabapentin; PMS-Gabapentin; Parke Davis brand of gabapentin; Pfizer brand of gabapentin; Pharmascience brand of gabapentin; Ratiopharm brand of gabapentin; Serada; Stadapharm brand of gabapentin; Vultin; Warner-Lambert brand of gabapentin; [1-(AMINOMETHYL)CYCLOHEXYL]ACETIC ACID
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Partial seizures [ICD11: 8A68.0] | Approved | [1] | |||
| Therapeutic Class |
Analgesics
|
|||||
| Structure |
|
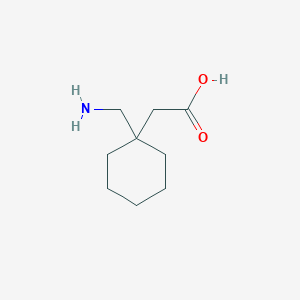 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C9H17NO2
|
|||||
| Canonical SMILES |
C1CCC(CC1)(CC(=O)O)CN
|
|||||
| InChI |
InChI=1S/C9H17NO2/c10-7-9(6-8(11)12)4-2-1-3-5-9/h1-7,10H2,(H,11,12)
|
|||||
| InChIKey |
UGJMXCAKCUNAIE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 60142-96-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 171.24 | Topological Polar Surface Area | 63.3 | ||
| Heavy Atom Count | 12 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 3 | |||
| XLogP |
-1.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321252
, 11111218
, 11112806
, 11113327
, 11466889
, 11468009
, 11486558
, 11528594
, 12012796
, 15171186
, 17405144
, 24278159
, 25623436
, 29222580
, 3206533
, 46506529
, 47291223
, 47365305
, 47515412
, 47589087
, 48035236
, 48413201
, 48416046
, 49655262
, 49698669
, 49833701
, 50100248
, 50103936
, 50103937
, 50519620
, 53777688
, 56312982
, 56365854
, 5651496
, 57321804
, 7847398
, 7887769
, 7979342
, 8028266
, 8028269
, 8152192
, 85174424
, 85209452
, 85231060
, 855579
, 87560508
, 89649772
, 90340599
, 91146476
, 92125867
|
|||||
| ChEBI ID |
ChEBI:42797
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | LAT1 | Transporter Info | L-type amino acid transporter 1 | Substrate | [2] | |
| OCT-2 | Transporter Info | Organic cation transporter 2 | Substrate | [3] | ||
| OCTN1 | Transporter Info | Organic cation/carnitine transporter 1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | LAT1 | Transporter Info | Km = 530 microM | hCMEC/D3 cells-LAT1 | [2] | |
| References | ||||||
| 1 | Gabapentin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol. 2013 Jun 1;85(11):1672-83. | |||||
| 3 | Clinical pharmacokinetic drug interaction studies of gabapentin enacarbil, a novel transported prodrug of gabapentin, with naproxen and cimetidine. Br J Clin Pharmacol. 2010 May;69(5):498-507. | |||||
| 4 | Effects of genetic variation in the novel organic cation transporter, OCTN1, on the renal clearance of gabapentin. Clin Pharmacol Ther. 2008 Mar;83(3):416-21. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
