Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00227
|
|||||
| Drug Name |
Rivaroxaban
|
|||||
| Synonyms |
XARELTO (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Prophylaxis of deep vein thrombosis [ICD11: BD71] | Approved | [1] | |||
| Structure |
|
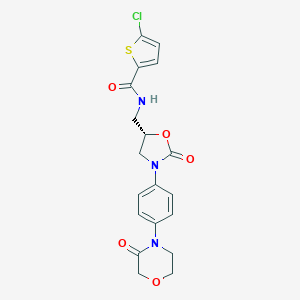 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H18ClN3O5S
|
|||||
| Canonical SMILES |
C1COCC(=O)N1C2=CC=C(C=C2)N3CC(OC3=O)CNC(=O)C4=CC=C(S4)Cl
|
|||||
| InChI |
InChI=1S/C19H18ClN3O5S/c20-16-6-5-15(29-16)18(25)21-9-14-10-23(19(26)28-14)13-3-1-12(2-4-13)22-7-8-27-11-17(22)24/h1-6,14H,7-11H2,(H,21,25)/t14-/m0/s1
|
|||||
| InChIKey |
KGFYHTZWPPHNLQ-AWEZNQCLSA-N
|
|||||
| CAS Number |
CAS 366789-02-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 435.9 | Topological Polar Surface Area | 116 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
2.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103469795
, 109693118
, 126659499
, 126666963
, 134342998
, 134358083
, 135213418
, 135684125
, 136375511
, 136920411
, 137128738
, 139479843
, 14856850
, 152258851
, 152344334
, 15279667
, 160645892
, 160647701
, 160969514
, 162172236
, 164045359
, 164194090
, 164824489
, 165245531
, 170498903
, 171578894
, 172091286
, 174477515
, 175266472
, 175427064
, 175610897
, 177748765
, 178103004
, 180189087
, 185985285
, 198991713
, 22395270
, 24164597
, 45622373
, 46513422
, 51091428
, 52936629
, 56373953
, 57373622
, 71825413
, 87225360
, 87557471
, 91615930
, 93580340
, 99444948
|
|||||
| ChEBI ID |
ChEBI:68579
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Rivaroxaban was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Absence of both MDR1 (ABCB1) and breast cancer resistance protein (ABCG2) transporters significantly alters rivaroxaban disposition and central nervous system entry. Basic Clin Pharmacol Toxicol. 2013 Mar;112(3):164-70. | |||||
| 3 | Downregulation of ABCB1 gene in patients with total hip or knee arthroplasty influences pharmacokinetics of rivaroxaban: a population pharmacokinetic-pharmacodynamic study. Eur J Clin Pharmacol. 2019 Feb 6. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
