Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00243
|
|||||
| Drug Name |
Mycophenolate mofetil
|
|||||
| Synonyms |
2-Morpholinoethyl (4E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoate; 2-Morpholinoethyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-5-phthalanyl)-4-methyl-4-hexenoate; 2-morpholin-4-ylethyl (4E)-6-[4-hydroxy-7-methyl-6-(methyloxy)-3-oxo-1,3-dihydro-2-benzofuran-5-yl]-4-methylhex-4-enoate; 2-morpholin-4-ylethyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-2-benzofuran-5-yl)-4-methylhex-4-enoate;4-Hexenoic acid, 6-(1,3-dihydro-4-hydroxy-6-methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-, 2-(4-morpholinyl)ethyl ester, (4E); CellCept; CellCept, RS 61443, TM-MMF, Mycophenolate mofetil; Cellcept (TN); ME-MPA; MMF; MMF CellCept(TM); Munoloc; Mycophenolate mofetil (JAN/USAN); Mycophenolic acid morpholinoethyl ester; Mycophenylate mofetil; R-99; RS 61443; RS-61443; RS-61443-190; TM-MMF
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Pemphigus vulgaris [ICD11: EB40.0] | Approved | [1] | |||
| Therapeutic Class |
Immunosuppressive Agents
|
|||||
| Structure |
|
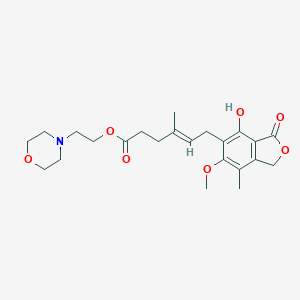 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C23H31NO7
|
|||||
| Canonical SMILES |
CC1=C2COC(=O)C2=C(C(=C1OC)CC=C(C)CCC(=O)OCCN3CCOCC3)O
|
|||||
| InChI |
InChI=1S/C23H31NO7/c1-15(5-7-19(25)30-13-10-24-8-11-29-12-9-24)4-6-17-21(26)20-18(14-31-23(20)27)16(2)22(17)28-3/h4,26H,5-14H2,1-3H3/b15-4+
|
|||||
| InChIKey |
RTGDFNSFWBGLEC-SYZQJQIISA-N
|
|||||
| CAS Number |
CAS 128794-94-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 433.5 | Topological Polar Surface Area | 94.5 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 10 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
3.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10110
, 103524891
, 103831140
, 104253178
, 113854519
, 117622077
, 118053002
, 12014254
, 124757298
, 125164102
, 125275456
, 126592936
, 126621236
, 126622394
, 126651543
, 126669998
, 126682700
, 131295332
, 131297141
, 132003527
, 134338068
, 134339833
, 135016986
, 135039794
, 136949095
, 137004579
, 142385187
, 144075766
, 14758955
, 29215410
, 39289999
, 46505626
, 48416299
, 49830660
, 49976609
, 50064190
, 537396
, 56310881
, 56312885
, 57358003
, 62129612
, 623972
, 77319688
, 7847817
, 80954261
, 8616507
, 92308999
, 92718908
, 93619754
, 96099842
|
|||||
| ChEBI ID |
ChEBI:93612
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Mycophenolic acid was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Influence of drug transporters and UGT polymorphisms on pharmacokinetics of phenolic glucuronide metabolite of mycophenolic acid in Japanese renal transplant recipients. Ther Drug Monit. 2008 Oct;30(5):559-64. | |||||
| 3 | Influence of SLCO1B1, 1B3, 2B1 and ABCC2 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol. 2007 Dec;63(12):1161-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
