Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00290
|
|||||
| Drug Name |
Lopinavir
|
|||||
| Synonyms |
A 157378; A 157378.0; A-157378-0; A-157378.0; ABT 157378; ABT 378; ABT-378; ABT-378, LOPINAVIR; AIDS032937; Aluvia (TN); Aluviran; Kaletra (TN); Koletra; LPV; Lopinavir (JAN/USAN/INN); Lopinavir [USAN:INN:BAN]; RS-346
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | |||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
| Structure |
|
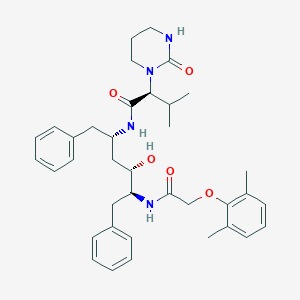 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C37H48N4O5
|
|||||
| Canonical SMILES |
CC1=C(C(=CC=C1)C)OCC(=O)NC(CC2=CC=CC=C2)C(CC(CC3=CC=CC=C3)NC(=O)C(C(C)C)N4CCCNC4=O)O
|
|||||
| InChI |
InChI=1S/C37H48N4O5/c1-25(2)34(41-20-12-19-38-37(41)45)36(44)39-30(21-28-15-7-5-8-16-28)23-32(42)31(22-29-17-9-6-10-18-29)40-33(43)24-46-35-26(3)13-11-14-27(35)4/h5-11,13-18,25,30-32,34,42H,12,19-24H2,1-4H3,(H,38,45)(H,39,44)(H,40,43)/t30-,31-,32-,34-/m0/s1
|
|||||
| InChIKey |
KJHKTHWMRKYKJE-SUGCFTRWSA-N
|
|||||
| CAS Number |
CAS 192725-17-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 628.8 | Topological Polar Surface Area | 120 | ||
| Heavy Atom Count | 46 | Rotatable Bond Count | 15 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
5.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10225535
, 103198077
, 104178992
, 104408196
, 11108093
, 11528784
, 123080504
, 124757206
, 124893167
, 125164010
, 126522368
, 126655919
, 126665841
, 127310211
, 127310212
, 127310213
, 127338643
, 127338644
, 127338645
, 134337997
, 135051036
, 136283935
, 136367896
, 14912304
, 14912305
, 26737285
, 26757998
, 44423527
, 46392556
, 46508588
, 49853992
, 50086984
, 50096471
, 50096475
, 50111692
, 53812811
, 53812974
, 56310583
, 57335304
, 583260
, 615079
, 76999753
, 7848488
, 7885634
, 7979791
, 826916
, 841952
, 87557375
, 87557376
, 99437252
|
|||||
| ChEBI ID |
ChEBI:31781
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Lopinavir was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | Inhibition of P-glycoprotein and multidrug resistance-associated proteins modulates the intracellular concentration of lopinavir in cultured CD4 T cells and primary human lymphocytes. J Antimicrob Chemother. 2007 Nov;60(5):987-93. | |||||
| 4 | Effects of cytochrome P450 3A (CYP3A) and the drug transporters P-glycoprotein (MDR1/ABCB1) and MRP2 (ABCC2) on the pharmacokinetics of lopinavir. Br J Pharmacol. 2010 Jul;160(5):1224-33. | |||||
| 5 | Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011 Mar;63(1):157-81. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
