Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00329
|
|||||
| Drug Name |
Glipizide
|
|||||
| Synonyms |
1-Cyclohexyl-3-((p-(2-(5-methylpyrazinecarboxamido)ethyl)phenyl)sulfonyl)urea; 1-Cyclohexyl-3-{4-[2-(5-methylpyrazine-2-carboxamido)ethyl]phenylsulfonyl}urea; Aldiab; Alphapharm Brand of Glipizide; CP 28,720; CP 28720; CP-28720; Digrin; Dipazide; G-117; Glibenese; Glibenese Brand of Glipizide; Glibetin; Glican; Glide; Glidiab; Glidiazinamide; Glipid; Glipizida; Glipizida [INN-Spanish]; Glipizide (USP/INN); Glipizide Extended-Release Tablets; Glipizide Kenfarma Brand; Glipizide [USAN:BAN:INN]; Glipizidum; Glipizidum [INN-Latin]; Gluco-Rite; Glucolip; Glucotrol; Glucotrol (TN); Glucotrol XL; Glucotrol XL, Glucotrol, Glipizide; Glucozide; Glupitel; Glupizide; Glyde; Glydiazinamide; Glypidizine; K 4024; K-4024; K4024; KS-1068; Kenfarma Brand of Glipizide; Lacer Brand of Glipizide; Lilly Brand of Glipizide; Melizide; Metaglip; Mindiab; Minidab; Minidiab; Minodiab; N-(2-{4-[(cyclohexylcarbamoyl)sulfamoyl]phenyl}ethyl)-5-methylpyrazine-2-carboxamide; N-(4-(beta-(5-Methylpyrazine-2-carboxamido)ethyl)benzenesulphonyl)-N'-cyclohexylurea; N-[2-(4-{[(cyclohexylcarbamoyl)amino]sulfonyl}phenyl)ethyl]-5-methylpyrazine-2-carboxamide; N-[2-[4-(cyclohexylcarbamoylsulfamoyl)phenyl]ethyl]-5-methylpyrazine-2-carboxamide; Napizide; Ozidia; Pfizer Brand 2 of Glipizide; PfizerBrand 1 of Glipizide; Samarium(III) ionophore I; Semiglynase; Sucrazide; TK 1320
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Diabetes [ICD11: 5A10-5A14] | Approved | [1] | |||
| Therapeutic Class |
Hypoglycemic Agents
|
|||||
| Structure |
|
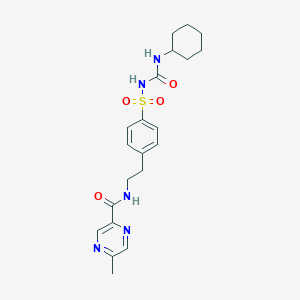 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H27N5O4S
|
|||||
| Canonical SMILES |
CC1=CN=C(C=N1)C(=O)NCCC2=CC=C(C=C2)S(=O)(=O)NC(=O)NC3CCCCC3
|
|||||
| InChI |
InChI=1S/C21H27N5O4S/c1-15-13-24-19(14-23-15)20(27)22-12-11-16-7-9-18(10-8-16)31(29,30)26-21(28)25-17-5-3-2-4-6-17/h7-10,13-14,17H,2-6,11-12H2,1H3,(H,22,27)(H2,25,26,28)
|
|||||
| InChIKey |
ZJJXGWJIGJFDTL-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 29094-61-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 445.5 | Topological Polar Surface Area | 139 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
1.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321342
, 11111214
, 11112714
, 11119955
, 11120443
, 11120931
, 11121414
, 11121894
, 11147038
, 11362483
, 11365045
, 11367607
, 11370237
, 11370238
, 11373208
, 11375769
, 11466159
, 11467279
, 11485877
, 14906210
, 17405183
, 24277839
, 24858438
, 25819918
, 26751569
, 26751570
, 29222611
, 3153201
, 46505865
, 47290874
, 47290875
, 47515059
, 47588733
, 47662000
, 47810481
, 47885136
, 49698363
, 49836250
, 50060598
, 50100247
, 50104189
, 50104190
, 50104191
, 5043710
, 53777727
, 53787230
, 7847401
, 7979413
, 8152212
, 855947
|
|||||
| ChEBI ID |
ChEBI:5384
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BSEP | Transporter Info | Bile salt export pump | Substrate | [2] | |
| References | ||||||
| 1 | Glipizide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Early identification of clinically relevant drug interactions with the human bile salt export pump (BSEP/ABCB11). Toxicol Sci. 2013 Dec;136(2):328-43. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
