Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00354
|
|||||
| Drug Name |
Afatinib
|
|||||
| Synonyms |
(2E)-N-{4-[(3-chloro-4-fluorophenyl)amino]-7-[(3S)-tetrahydrofuran-3-yloxy]quinazolin-6-yl}-4-(dimethylamino)but-2-enamide; BIBW 2992; BIBW-2992; EGFR inhibitor 2nd gens; Tomtovok; Tovok; Tovok (TN); Tovok, BIBW2992
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Non-small cell lung cancer [ICD11: 2C25] | Approved | [1] | |||
| Structure |
|
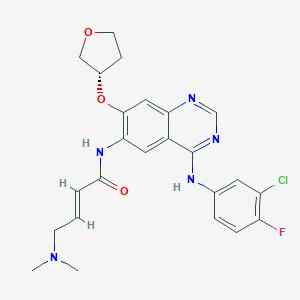 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H25ClFN5O3
|
|||||
| Canonical SMILES |
CN(C)CC=CC(=O)NC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC(=C(C=C3)F)Cl)OC4CCOC4
|
|||||
| InChI |
InChI=1S/C24H25ClFN5O3/c1-31(2)8-3-4-23(32)30-21-11-17-20(12-22(21)34-16-7-9-33-13-16)27-14-28-24(17)29-15-5-6-19(26)18(25)10-15/h3-6,10-12,14,16H,7-9,13H2,1-2H3,(H,30,32)(H,27,28,29)/b4-3+/t16-/m0/s1
|
|||||
| InChIKey |
ULXXDDBFHOBEHA-CWDCEQMOSA-N
|
|||||
| CAS Number |
CAS 850140-72-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 485.9 | Topological Polar Surface Area | 88.6 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103768626
, 104144352
, 111978343
, 118049494
, 123051125
, 124490464
, 124756933
, 124896577
, 125163740
, 125329929
, 126578844
, 126666994
, 126726460
, 131407778
, 131465104
, 134222879
, 134339089
, 135257273
, 135267496
, 135727456
, 136368046
, 136920296
, 137039955
, 137263401
, 142681900
, 143499145
, 144072463
, 144115710
, 15180141
, 151990100
, 152043638
, 152234944
, 152258159
, 152344140
, 160646996
, 162011458
, 162037384
, 162202549
, 163404060
, 163406016
, 164041641
, 164825245
, 164835145
, 165245589
, 170502166
, 22579524
, 40274223
, 57304398
, 78996079
, 99432363
|
|||||
| ChEBI ID |
CHEBI:61390
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Afatinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Breast cancer resistance protein (BCRP/ABCG2) and P-glycoprotein (P-gp/ABCB1) transport afatinib and restrict its oral availability and brain accumulation. Pharmacol Res. 2017 Jun;120:43-50. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
