Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00386
|
|||||
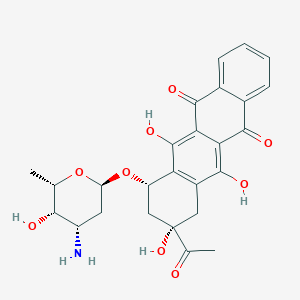
| Drug Name |
Idarubicin
|
|||||
| Synonyms |
(1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (1s,3s)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-A-l-lyxo-hexopyranoside; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S-cis)-9-Acetyl-7-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-5,12-naphthacenedione; 4-DMD; 4-Demethoxydaunomycin; 4-Demethoxydaunorubicin; 4-Desmethoxydaunorubicin; DM5; DMDR; I 1656; IMI 30; IMI-30; Idamycin; Idamycin (TN); Idarubicin (INN); Idarubicin Hcl; Idarubicin Hcl Pfs; Idarubicin [INN:BAN]; Idarubicin hydrochloride; Idarubicina; Idarubicina [INN-Spanish]; Idarubicine; Idarubicine [INN-French]; Idarubicinum; Idarubicinum [INN-Latin]; Zavedos; Zavedos (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acute myeloid leukemia [ICD11: 2A60] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H27NO9
|
|||||
| Canonical SMILES |
CC1C(C(CC(O1)OC2CC(CC3=C2C(=C4C(=C3O)C(=O)C5=CC=CC=C5C4=O)O)(C(=O)C)O)N)O
|
|||||
| InChI |
InChI=1S/C26H27NO9/c1-10-21(29)15(27)7-17(35-10)36-16-9-26(34,11(2)28)8-14-18(16)25(33)20-19(24(14)32)22(30)12-5-3-4-6-13(12)23(20)31/h3-6,10,15-17,21,29,32-34H,7-9,27H2,1-2H3/t10-,15-,16-,17-,21+,26-/m0/s1
|
|||||
| InChIKey |
XDXDZDZNSLXDNA-TZNDIEGXSA-N
|
|||||
| CAS Number |
CAS 58957-92-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 497.5 | Topological Polar Surface Area | 177 | ||
| Heavy Atom Count | 36 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 10 | |||
| XLogP |
1.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103311042
, 104340903
, 11378086
, 117597729
, 117695696
, 121361286
, 124749845
, 124891657
, 126626724
, 126661729
, 127301008
, 127301009
, 127301010
, 127301011
, 127301012
, 127301013
, 127301014
, 127301015
, 127301016
, 127301017
, 127301018
, 14810780
, 14908709
, 17405162
, 24278488
, 26697284
, 26704230
, 26704281
, 34708383
, 46506973
, 47959947
, 48416101
, 49995025
, 50106402
, 50106403
, 50106404
, 53777706
, 53788391
, 57312753
, 7887077
, 794734
, 7979585
, 79820214
, 8177897
, 85789484
, 87325144
, 90340569
, 92303774
, 92308741
, 96024754
|
|||||
| ChEBI ID |
ChEBI:42068
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Idarubicin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Amonafide L-malate is not a substrate for multidrug resistance proteins in secondary acute myeloid leukemia. Leukemia. 2008 Nov;22(11):2110-5. | |||||
| 3 | Human intestinal transporter database: QSAR modeling and virtual profiling of drug uptake, efflux and interactions. Pharm Res. 2013 Apr;30(4):996-1007. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
