Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00391
|
|||||
| Drug Name |
Cerivastatin
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hyperlipidaemia [ICD11: 5C8Z] | Approved | [1] | |||
| Structure |
|
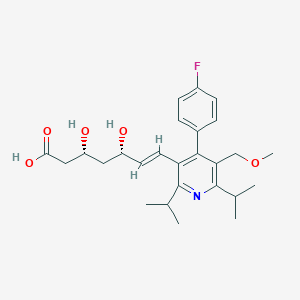 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H34FNO5
|
|||||
| Canonical SMILES |
CC(C)C1=C(C(=C(C(=N1)C(C)C)COC)C2=CC=C(C=C2)F)C=CC(CC(CC(=O)O)O)O
|
|||||
| InChI |
InChI=1S/C26H34FNO5/c1-15(2)25-21(11-10-19(29)12-20(30)13-23(31)32)24(17-6-8-18(27)9-7-17)22(14-33-5)26(28-25)16(3)4/h6-11,15-16,19-20,29-30H,12-14H2,1-5H3,(H,31,32)/b11-10+/t19-,20-/m1/s1
|
|||||
| InChIKey |
SEERZIQQUAZTOL-ANMDKAQQSA-N
|
|||||
| CAS Number |
CAS 145599-86-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 459.5 | Topological Polar Surface Area | 99.9 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
3.6
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10167
, 10299839
, 103541455
, 104635877
, 10852026
, 126681828
, 134339434
, 134339836
, 135259857
, 135650063
, 137002687
, 142742086
, 14809054
, 14858094
, 160963785
, 164788182
, 164807583
, 176484089
, 179116613
, 184546299
, 198953954
, 223659548
, 223898596
, 226406536
, 226406537
, 241035409
, 252347182
, 36888654
, 46505877
, 46518451
, 48415746
, 50067077
, 50070698
, 50728881
, 51091965
, 56312092
, 56313141
, 56313595
, 7978896
, 822164
, 92308707
, 93166525
, 93578306
|
|||||
| ChEBI ID |
CHEBI:3558
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| NTCP | Transporter Info | Sodium/taurocholate cotransporting polypeptide | Substrate | [4] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [5] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [5] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Cerivastatin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | Identification of the hepatic efflux transporters of organic anions using double-transfected Madin-Darby canine kidney II cells expressing human organic anion-transporting polypeptide 1B1 (OATP1B1)/multidrug resistance-associated protein 2, OATP1B1/multidrug resistance 1, and OATP1B1/breast cancer resistance protein. J Pharmacol Exp Ther. 2005 Sep;314(3):1059-67. | |||||
| 4 | Differential effect of genetic variants of Na(+)-taurocholate co-transporting polypeptide (NTCP) and organic anion-transporting polypeptide 1B1 (OATP1B1) on the uptake of HMG-CoA reductase inhibitors. Xenobiotica. 2011 Jan;41(1):24-34. | |||||
| 5 | FDA Drug Development and Drug Interactions | |||||
| 6 | pH-sensitive interaction of HMG-CoA reductase inhibitors (statins) with organic anion transporting polypeptide 2B1. Mol Pharm. 2011 Aug 1;8(4):1303-13. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
