Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00423
|
|||||
| Drug Name |
Gefitinib
|
|||||
| Synonyms |
4-(3'-Chloro-4'-fluoroanilino)-7-methoxy-6-(3-morpholinopropoxy)quinazoline; 6-(3-morpholinopropoxy)-N-(3-chloro-4-fluorophenyl)-7-methoxyquinazolin-4-amine; CU-00000000396-1; Gefitini; Gefitinib (JAN/USAN/INN); Gefitinib [USAN]; Gefitinib,Iressa, ZD1839; IRE; Iressa; Iressa (TN); Iressa(TM); Irressat; N-(3-Chloro-4-fluoro-phenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine; N-(3-Chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholinopropoxy)quinazolin-4-amine; N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-(4-morpholinyl)propoxy)-4-quinazolinamide; N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholin-4-ylpropoxy)quinazolin-4-amine; N-(3-chloro-4-fluorophenyl)-7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4-amine; ZD 1839; ZD-1839; ZD-1839, Iressa, Gefitinib; ZD1839
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Urethral cancer [ICD11: 2F78] | Approved | [1] | ||||
| Non-small cell lung cancer [ICD11: 2C25] | Approved | [1] | ||||
| Ovarian cancer [ICD11: 2C73] | Preclinical | [1] | ||||
| Prostate cancer [ICD11: 2C82] | Preclinical | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
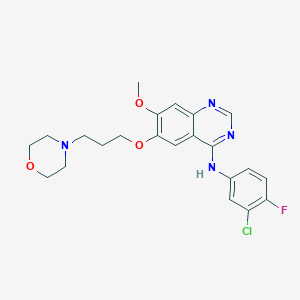 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H24ClFN4O3
|
|||||
| Canonical SMILES |
COC1=C(C=C2C(=C1)N=CN=C2NC3=CC(=C(C=C3)F)Cl)OCCCN4CCOCC4
|
|||||
| InChI |
InChI=1S/C22H24ClFN4O3/c1-29-20-13-19-16(12-21(20)31-8-2-5-28-6-9-30-10-7-28)22(26-14-25-19)27-15-3-4-18(24)17(23)11-15/h3-4,11-14H,2,5-10H2,1H3,(H,25,26,27)
|
|||||
| InChIKey |
XGALLCVXEZPNRQ-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 184475-35-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 446.9 | Topological Polar Surface Area | 68.7 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
4.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10240807
, 103024897
, 10318995
, 103244983
, 103905343
, 103905344
, 104418951
, 104829175
, 11377941
, 117865087
, 118047033
, 12015033
, 123105108
, 124756943
, 124892204
, 124892205
, 124892206
, 125001914
, 14833109
, 21317853
, 24424016
, 24424024
, 24424026
, 29215403
, 29215404
, 29303859
, 46508649
, 47646567
, 49635529
, 49742641
, 50040863
, 50100103
, 532631
, 53319998
, 53788589
, 53799235
, 56312091
, 56313236
, 56313469
, 57340492
, 61127928
, 7849039
, 8035066
, 81092810
, 85171051
, 92308715
, 92717784
, 93581024
, 99436946
, 99444469
|
|||||
| ChEBI ID |
ChEBI:49668
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [3] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Gefitinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013 Mar 15;19(6):1458-66. | |||||
| 4 | Gefitinib-phenytoin interaction is not correlated with the C-erythromycin breath test in healthy male volunteers. Br J Clin Pharmacol. 2009 Aug;68(2):226-37. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
