Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00448
|
|||||
| Drug Name |
Cyclosporine
|
|||||
| Synonyms |
cyclosporin A; cyclosporine; Ciclosporin; Cyclosporine A; Ciclosporine; Neoral; Cyclosporin; Ciclosporinum; Ciclosporina; Sandimmune; Equoral; Neoplanta; Sandimmun; Sang-35; Gengraf; Sandimmun Neoral; UNII-83HN0GTJ6D; Antibiotic S 7481F1; Consupren; Restasis; Ramihyphin A; SangCyA; MFCD00274558; 83HN0GTJ6D; MLS001333756; CSA; S-Neoral; Cipol N; Sigmasporin Microoral; Sang 35; DSSTox_CID_365; Ciclosporinum [INN-Latin]; Ciclosporine [INN-French]; Ciclosporina [INN-Spanish]; DSSTox_RID_75541; Ciclosporin (Ciclosporin A)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Xerophthalmia [ICD11: 5B55.Y] | Approved | [1] | |||
| Therapeutic Class |
Immunosuppressive Agents
|
|||||
| Structure |
|
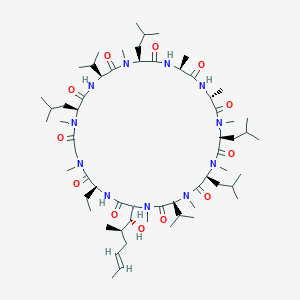 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C62H111N11O12
|
|||||
| Canonical SMILES |
CCC1C(=O)N(CC(=O)N(C(C(=O)NC(C(=O)N(C(C(=O)NC(C(=O)NC(C(=O)N(C(C(=O)N(C(C(=O)N(C(C(=O)N(C(C(=O)N1)C(C(C)CC=CC)O)C)C(C)C)C)CC(C)C)C)CC(C)C)C)C)C)CC(C)C)C)C(C)C)CC(C)C)C)C
|
|||||
| InChI |
InChI=1S/C62H111N11O12/c1-25-27-28-40(15)52(75)51-56(79)65-43(26-2)58(81)67(18)33-48(74)68(19)44(29-34(3)4)55(78)66-49(38(11)12)61(84)69(20)45(30-35(5)6)54(77)63-41(16)53(76)64-42(17)57(80)70(21)46(31-36(7)8)59(82)71(22)47(32-37(9)10)60(83)72(23)50(39(13)14)62(85)73(51)24/h25,27,34-47,49-52,75H,26,28-33H2,1-24H3,(H,63,77)(H,64,76)(H,65,79)(H,66,78)/b27-25+/t40-,41+,42-,43+,44+,45+,46+,47+,49+,50+,51+,52-/m1/s1
|
|||||
| InChIKey |
PMATZTZNYRCHOR-CGLBZJNRSA-N
|
|||||
| CAS Number |
CAS 59865-13-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 1202.6 | Topological Polar Surface Area | 279 | ||
| Heavy Atom Count | 85 | Rotatable Bond Count | 15 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 12 | |||
| XLogP |
7.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
127280714
, 127280715
, 127280716
, 127280717
, 127280718
, 127280719
, 127280720
, 127280721
, 127280722
, 127280723
, 127280724
, 127280725
, 127280726
, 127280727
, 127280728
, 127280729
, 127280730
, 127280731
, 127280732
, 127300898
, 127300899
, 127300900
, 127300901
, 127300902
, 127300903
, 127300904
, 127300905
, 127300906
, 127300907
, 127300908
, 127300909
, 127300910
, 127300911
, 127300912
, 127300913
, 127300914
, 137018976
, 226407640
|
|||||
| ChEBI ID |
ChEBI:4031
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km = 0.17 microM | High five cells-MDR1 | [5] | |
| P-GP | Transporter Info | Km = 3.8 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [6] | ||
| P-GP | Transporter Info | Km = 8.4 microM | LLC-PK1 cells-MDR1 | [7] | ||
| References | ||||||
| 1 | Cyclosporine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | Is cyclosporine A transport inhibited by pravastatin via multidrug resistant protein 2? Eur J Clin Pharmacol. 2010 Feb;66(2):153-8. | |||||
| 4 | Contribution of down-regulation of intestinal and hepatic cytochrome P450 3A to increased absorption of cyclosporine A in a rat nephrosis model. J Pharmacol Exp Ther. 2008 Nov;327(2):592-9. | |||||
| 5 | Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-glycoprotein. Pharm Res. 2001 Dec;18(12):1660-8. | |||||
| 6 | Relevance of p-glycoprotein for the enteral absorption of cyclosporin A: in vitro-in vivo correlation. Br J Pharmacol. 1996 Aug;118(7):1841-7. Clinical Trial | |||||
| 7 | Human P-glycoprotein transports cyclosporin A and FK506. J Biol Chem. 1993 Mar 25;268(9):6077-80. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
