Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00458
|
|||||
| Drug Name |
Pantoprazole
|
|||||
| Synonyms |
5-(Difluoromethoxy)-2-(((3,4-dimethoxy-2-pyridyl)methyl)sulfinyl)benzimidazole; 5-(difluoromethoxy)-2-{[(3,4-dimethoxypyridin-2-yl)methyl]sulfinyl}-1H-benzimidazole; 6-(difluoromethoxy)-2-[(3,4-dimethoxypyridin-2-yl)methylsulfinyl]-1H-benzimidazole; Astropan; Astropan (TN); BY 1023; BY-1023; Controloc (TN); Inipomp (TN); Pantecta (TN); Pantoloc (TN); Pantopan (TN); Pantoprazol; Pantoprazol [INN-Spanish]; Pantoprazole (USAN/INN); Pantoprazole Na; Pantoprazole Sodium; Pantoprazole [USAN:BAN:INN]; Pantoprazolum; Pantoprazolum [INN-Latin]; Pantoprozole; Pantor; Pantor (TN); Pantotab (TN); Pantozol; Pantozol (TN); Protium; Protium (TN); Protonix; Protonix (TN); Protonix IV; SK&F 96022; SK&F-96022; SK-96022; SKF-96022; Somac; Somac (TN); Ulcepraz (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gastroesophageal reflux disease [ICD11: DA22] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
| Structure |
|
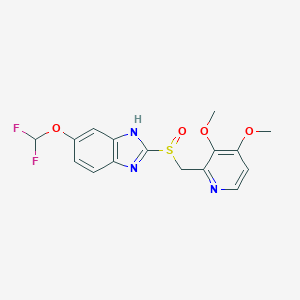 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C16H15F2N3O4S
|
|||||
| Canonical SMILES |
COC1=C(C(=NC=C1)CS(=O)C2=NC3=C(N2)C=C(C=C3)OC(F)F)OC
|
|||||
| InChI |
InChI=1S/C16H15F2N3O4S/c1-23-13-5-6-19-12(14(13)24-2)8-26(22)16-20-10-4-3-9(25-15(17)18)7-11(10)21-16/h3-7,15H,8H2,1-2H3,(H,20,21)
|
|||||
| InChIKey |
IQPSEEYGBUAQFF-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 102625-70-7
|
|||||
| Pharmaceutical Properties | Molecular Weight | 383.4 | Topological Polar Surface Area | 106 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 9 | |||
| XLogP |
2.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103557047
, 104098729
, 104178843
, 104307108
, 118318262
, 125336751
, 126525315
, 126630995
, 126658184
, 126670715
, 128206212
, 131293114
, 134338012
, 135028043
, 135692424
, 136948045
, 137088311
, 137175473
, 13970
, 141641906
, 141641915
, 142318542
, 14902603
, 17183107
, 17398288
, 26526442
, 26612946
, 26749082
, 26749083
, 29223767
, 46504622
, 47959868
, 48416375
, 49830151
, 49882833
, 50107519
, 50432438
, 53788377
, 56394975
, 57322393
, 6752164
, 7980248
, 81040904
, 85789648
, 91011863
, 92124724
, 92307944
, 92309139
, 92711310
, 93166161
|
|||||
| ChEBI ID |
ChEBI:7915
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Pantoprazole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004 Aug 15;64(16):5804-11. | |||||
| 3 | A novel screening strategy to identify ABCB1 substrates and inhibitors. Naunyn Schmiedebergs Arch Pharmacol. 2009 Jan;379(1):11-26. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
