Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00558
|
|||||
| Drug Name |
Erlotinib
|
|||||
| Synonyms |
4-[(3-Ethynylphenyl)amino]-6,7-bis(2-methoxyethoxy)quinazoline; AQ4; CP 358,774; CP-358,774; CP-358774; Erlotinib Base; Erlotinib(Tarceva); Erlotinib, OS-774; Erlotinin; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine; N-(3-ethynylphenyl)[6,7-bis(2-methoxyethoxy)quinazolin-4-yl]amine; OSI 744; R 1415; Tarceva; Tarceva (TN); [6,7-BIS(2-METHOXY-ETHOXY)QUINAZOLINE-4-YL]-(3-ETHYNYLPHENYL)AMINE; [6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-ethynyl-phenyl)-amine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Non-small cell lung cancer [ICD11: 2C25] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
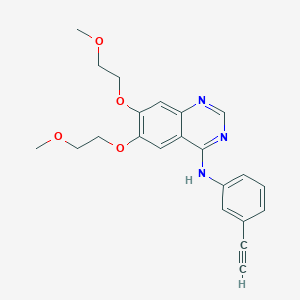
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H23N3O4
|
|||||
| Canonical SMILES |
COCCOC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC=CC(=C3)C#C)OCCOC
|
|||||
| InChI |
InChI=1S/C22H23N3O4/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25)
|
|||||
| InChIKey |
AAKJLRGGTJKAMG-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 183321-74-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 393.4 | Topological Polar Surface Area | 74.7 | ||
| Heavy Atom Count | 29 | Rotatable Bond Count | 11 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
3.3
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10258448
, 103177459
, 103905339
, 104425943
, 104831334
, 117695448
, 117866919
, 118049695
, 124893165
, 124893166
, 125001915
, 125343507
, 126622002
, 126653679
, 126670959
, 126670960
, 127301426
, 127301427
, 127301428
, 127301429
, 127301430
, 127301431
, 127301432
, 127301433
, 127301434
, 14720343
, 14878691
, 21317842
, 26757996
, 33500421
, 46508021
, 47957324
, 50068213
, 50100099
, 50405936
, 53788276
, 57395323
, 6593089
, 68530774
, 7885946
, 8035065
, 826174
, 85171065
, 85261833
, 85267493
, 87350514
, 92308781
, 92717787
, 93581027
, 94568883
|
|||||
| ChEBI ID |
CHEBI:114785
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Erlotinib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev. 2009 Jan 31;61(1):26-33. | |||||
| 3 | Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1-/-/Mdr1a/1b-/- (triple-knockout) and wild-type mice. Mol Cancer Ther. 2008 Aug;7(8):2280-7. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
