Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00619
|
|||||
| Drug Name |
Delavirdine
|
|||||
| Synonyms |
(1-(5-METHANSULPHONAMIDO-1H-INDOL-2-YL-CARBONYL)4-[METHYLAMINO)PYRIDINYL]PIPERAZINE; (N-[2-[4-[3-(1-methylethylamino)pyridin-2-yl]piperazin-1-yl]carbonyl-1H-indol-5-yl] methanesulfonamide); 1-(3-((1-Methylethyl)amino)-2-pyridinyl)-4-((5-((methylsulfonyl)amino)-1H-indol-2-yl)carbonyl)piperazine; 1-(5-Methanesulphonamido)-1H-indol-2-yl-carbonyl)-4-[3-(isopropylamino)-2-pyridinyl]piperaz; 2-(4-(5-Methanesulfonamido-1H-indol-2-ylcarbonyl)-1-piperazinyl)-N-(1-methylethyl)-3-pyridinamine; BHAP-U 90152; DELAVIRDINE MESYLATE; DLV; Delavirdine (*Mesylate salt*); Delavirdine (INN); Delavirdine [INN]; Delavirdine(U-90152) & .a.IFN; N-(2-(1-(3-(isopropylamino)pyridin-2-yl)piperazine-4-carbonyl)-1H-indol-5-yl)methanesulfonamide; N-[2-({4-[3-(propan-2-ylamino)pyridin-2-yl]piperazin-1-yl}carbonyl)-1H-indol-5-yl]methanesulfonamide; N-[2-[4-[3-(propan-2-ylamino)pyridin-2-yl]piperazine-1-carbonyl]-1H-indol-5-yl]methanesulfonamide; N-{2-[(4-{3-[(1-methylethyl)amino]pyridin-2-yl}piperazin-1-yl)carbonyl]-1H-indol-5-yl}methanesulfonamide; N-{2-[4-(3-Isopropylamino-pyridin-2-yl)-piperazine-1-carbonyl]-1H-indol-5-yl}-methanesulfonamide; PNU-90152-T; Piperazine, 1-[3-[(1-methylethyl)amino]-2-pyridinyl]-4-[[5-[(methylsulfonyl)amino]-1H-indol-2-yl]carbonyl]-& alpha-Interferon; Rescriptor; Rescriptor (TM);Rescriptor (TN); SPP; U 90152; U-90152; U-90152S; U90152S (*Mesylate salt*)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | |||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
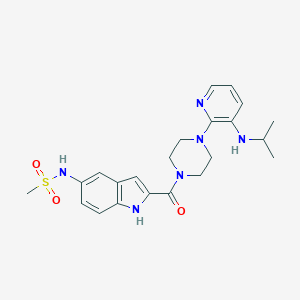
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C22H28N6O3S
|
|||||
| Canonical SMILES |
CC(C)NC1=C(N=CC=C1)N2CCN(CC2)C(=O)C3=CC4=C(N3)C=CC(=C4)NS(=O)(=O)C
|
|||||
| InChI |
InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3
|
|||||
| InChIKey |
WHBIGIKBNXZKFE-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 136817-59-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 456.6 | Topological Polar Surface Area | 119 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
2.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103181784
, 104309776
, 124893554
, 125822848
, 126653075
, 126666446
, 129580604
, 134337503
, 134358669
, 135022528
, 137006542
, 142315847
, 144206190
, 14857955
, 152104495
, 160964049
, 163122160
, 163693522
, 164788294
, 170464664
, 172913431
, 172919202
, 175267271
, 176484193
, 179149870
, 184546189
, 196107220
, 198992860
, 204376689
, 29224663
, 46508878
, 4700507
, 48415852
, 50064377
, 50113278
, 50860318
, 53788602
, 57322873
, 57580870
, 600721
, 633105
, 7890564
, 7979035
, 8031779
, 8153462
, 87350530
, 9156
, 92308796
, 96024480
, 99445034
|
|||||
| ChEBI ID |
CHEBI:119573
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [3] | ||
| References | ||||||
| 1 | Delavirdine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 3 | The transport of anti-HIV drugs across blood-CNS interfaces: summary of current knowledge and recommendations for further research. Antiviral Res. 2009 May;82(2):A99-109. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
