Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00620
|
|||||
| Drug Name |
Pramipexole
|
|||||
| Synonyms |
(-)-Pramipexole; (6S)-6-N-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine; (6S)-N(6)-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine; (6S)-N6-propyl-4,5,6,7-tetrahydro-1,3-benzothiazole-2,6-diamine; (S)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole; 111GE001; 2-amino-4,5,6,7-tetrahydro-6-propylaminobenzothiazole; 2-amino-6-propylaminotetrahydrobenzothiazole; Mirapex; Mirapex (TN); Mirapexin (TN); Pramipexol; Pramipexole (USAN/INN); Pramipexole [USAN:INN]; Pramipexolum; SND-919; SUD919CL2Y; Sifrol(TN); U-98528E
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Parkinson's Disease [ICD11: 8A00.0] | Approved | [1] | |||
| Therapeutic Class |
Antiparkinson Agents
|
|||||
| Structure |
|
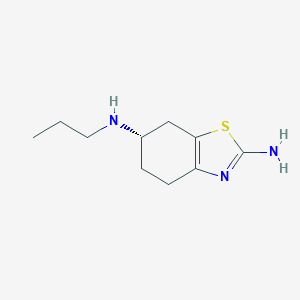 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C10H17N3S
|
|||||
| Canonical SMILES |
CCCNC1CCC2=C(C1)SC(=N2)N
|
|||||
| InChI |
InChI=1S/C10H17N3S/c1-2-5-12-7-3-4-8-9(6-7)14-10(11)13-8/h7,12H,2-6H2,1H3,(H2,11,13)/t7-/m0/s1
|
|||||
| InChIKey |
FASDKYOPVNHBLU-ZETCQYMHSA-N
|
|||||
| CAS Number |
CAS 104632-26-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 211.33 | Topological Polar Surface Area | 79.2 | ||
| Heavy Atom Count | 14 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
1.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103245538
, 104010405
, 104408638
, 10633207
, 11342091
, 11362274
, 11364752
, 11367314
, 11369876
, 11378041
, 11487676
, 11495652
, 118317775
, 124658831
, 124800011
, 127818967
, 131293470
, 135017226
, 135650847
, 137055231
, 137205527
, 142970931
, 144205998
, 14748917
, 14797724
, 160831530
, 162038142
, 163094982
, 163392621
, 164216289
, 26719739
, 29300417
, 46386856
, 46505897
, 47207239
, 47589086
, 48416455
, 49681584
, 49890661
, 50112695
, 56464131
, 57309747
, 57339564
, 75921463
, 7980372
, 91615696
, 92308251
, 92309051
, 93166408
, 99431914
|
|||||
| ChEBI ID |
ChEBI:8356
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [2] | |
| OCT-2 | Transporter Info | Organic cation transporter 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Pramipexole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | OCT1 polymorphism is associated with response and survival time in anti-Parkinsonian drug users. Neurogenetics. 2011 Feb;12(1):79-82. | |||||
| 3 | Uptake of pramipexole by human organic cation transporters. Mol Pharm. 2010 Aug 2;7(4):1342-7. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
