Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00638
|
|||||
| Drug Name |
Grepafloxacin
|
|||||
| Synonyms |
(+-)-1-Cyclopropyl-6-fluoro-1,4-dihydro-5-methyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-Cyclopropyl-6-fluoro-1,4-dihydro-5-methyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid; 1-cyclopropyl-6-fluoro-5-methyl-7-(3-methylpiperazin-1-yl)-4-oxoquinoline-3-carboxylic acid; Grepafloxacin (unspecified); Grepafloxacin [INN]; Raxar; Raxar (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Chronic bronchitis [ICD11: CA20.1] | Approved | [1] | |||
| Therapeutic Class |
Antibiotics
|
|||||
| Structure |
|
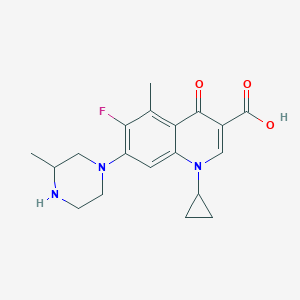 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H22FN3O3
|
|||||
| Canonical SMILES |
CC1CN(CCN1)C2=C(C(=C3C(=C2)N(C=C(C3=O)C(=O)O)C4CC4)C)F
|
|||||
| InChI |
InChI=1S/C19H22FN3O3/c1-10-8-22(6-5-21-10)15-7-14-16(11(2)17(15)20)18(24)13(19(25)26)9-23(14)12-3-4-12/h7,9-10,12,21H,3-6,8H2,1-2H3,(H,25,26)
|
|||||
| InChIKey |
AIJTTZAVMXIJGM-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 119914-60-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 359.4 | Topological Polar Surface Area | 72.9 | ||
| Heavy Atom Count | 26 | Rotatable Bond Count | 3 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
-0.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103179577
, 104011329
, 104353024
, 124766026
, 125536480
, 127411146
, 134338274
, 135029665
, 13542
, 137044453
, 143019201
, 14852426
, 160963712
, 162793470
, 163693542
, 163852853
, 179148061
, 184545887
, 184546334
, 223439875
, 224916848
, 226420671
, 241036011
, 242059760
, 43128629
, 46507253
, 50042505
, 50112780
, 50471882
, 53788063
, 57318735
, 603312
, 6610845
, 8195206
|
|||||
| ChEBI ID |
CHEBI:5543
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [4] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [6] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km = 580 microM | Human enterocyte-like 2 cells (Caco-2)-MDR1 | [7] | |
| References | ||||||
| 1 | Grepafloxacin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab Dispos. 2007 Oct;35(10):1873-9. | |||||
| 3 | Limited distribution of new quinolone antibacterial agents into brain caused by multiple efflux transporters at the blood-brain barrier. J Pharmacol Exp Ther. 2000 Oct;295(1):146-52. | |||||
| 4 | Fluoroquinolone efflux mediated by ABC transporters. J Pharm Sci. 2008 Sep;97(9):3483-93. | |||||
| 5 | Identification of influx transporter for the quinolone antibacterial agent levofloxacin. Mol Pharm. 2007 Jan-Feb;4(1):85-94. | |||||
| 6 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 7 | Secretory mechanisms of grepafloxacin and levofloxacin in the human intestinal cell line caco-2. J Pharmacol Exp Ther. 2000 Oct;295(1):360-6. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
