Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00646
|
|||||
| Drug Name |
Chlorothiazide
|
|||||
| Synonyms |
2H-1,2,4-Benzothiadiazine-7-sulfonamide, 6-chloro-, 1,1-dioxide; 6-Chloro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; 6-Chloro-7-sulfamoyl-2H-1,2,4-benzothiadiazine 1,1-dioxide; 6-chloro-4H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide; Aldoclor; Alurene; C 4911; Chloriazid; Chlorosal; Chlorothiazid; Chlorothiazide (JAN/USP/INN); Chlorothiazide [USAN:INN:BAN]; Chlorothiazidum; Chlorothiazidum [INN-Latin]; Chlorotiazida; Chlorthiazid; Chlorthiazide; Chlorthiazidum; Chlortiazid; Chlorurit; Chlotride; Chlrosal; Clorotiazida; Clorotiazida [INN-Spanish]; Clorotiazide; Clorotiazide [DCIT]; Clotride; Component of Aldoclor; Diupres; Diuresal; Diuril; Diuril (TN); Diuril Boluses; Diuril Boluses, Veterinary; Diuril, Chlotride, Chlorothiazide; Diurilix; Diurite; Diutrid; Flumen; Minzil; Neo-Dema; Salisan; Salunil; Saluretil; Saluric; Sk-chlorothiazide; Thiazide; Urinex; Warduzide; Yadalan
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Edema associated with congestive heart failure [ICD11: BD10] | Approved | [1] | |||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
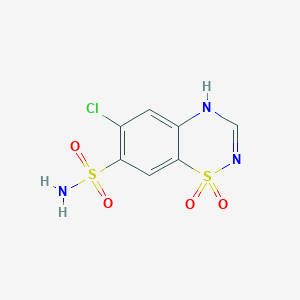
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C7H6ClN3O4S2
|
|||||
| Canonical SMILES |
C1=C2C(=CC(=C1Cl)S(=O)(=O)N)S(=O)(=O)N=CN2
|
|||||
| InChI |
InChI=1S/C7H6ClN3O4S2/c8-4-1-5-7(2-6(4)16(9,12)13)17(14,15)11-3-10-5/h1-3H,(H,10,11)(H2,9,12,13)
|
|||||
| InChIKey |
JBMKAUGHUNFTOL-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 58-94-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 295.7 | Topological Polar Surface Area | 135 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 1 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
-0.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321157
, 10528492
, 11110950
, 11110951
, 11335850
, 11361089
, 11363008
, 11365570
, 11368132
, 11371323
, 11373919
, 11376294
, 11462061
, 11466279
, 11467399
, 11484024
, 11486017
, 11487970
, 11490158
, 11492095
, 11493948
, 15023005
, 17389544
, 17404817
, 22425567
, 24278150
, 24714733
, 26611653
, 26680067
, 26747285
, 26747286
, 29221877
, 46507032
, 47291140
, 47291141
, 47291142
, 47515323
, 47515324
, 47662287
, 47885422
, 47959750
, 48415760
, 4970936
, 5051008
, 542598
, 7847585
, 7978922
, 8149249
, 855976
, 9664
|
|||||
| ChEBI ID |
CHEBI:3640
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [3] | ||
| References | ||||||
| 1 | Chlorothiazide was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | ABCG2 modulates chlorothiazide permeability--in vitro-characterization of its interactions. Drug Metab Pharmacokinet. 2012;27(3):349-53. | |||||
| 3 | Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. Am J Physiol Renal Physiol. 2008 Apr;294(4):F867-73. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
