Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00693
|
|||||
| Drug Name |
Prazosin
|
|||||
| Synonyms |
1-(3-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanyl-carbonyl)piperazine hydrochloride; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl) piperazine; 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine; 2-(4-(2-Furoyl)piperazin-1-yl)-4-amino-6,7-dimethoxyquinazoline; 2-[4-(2-furoyl)piperazin-1-yl]-6,7-dimethoxyquinazolin-4-amine; 4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazinyl 2-furyl ketone; CP-12299; Furazosin; Hypovase (TN); Justac; Lentopres; Minipress (TN); Piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)-(8CI); Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)-(9CI); Prazocin; Prazosin (INN); Prazosin HCl; Prazosin [INN:BAN]; Prazosina; Prazosina [INN-Spanish]; Prazosine; Prazosine [INN-French]; Prazosinum; Prazosinum [INN-Latin]; TNP00312; Vasoflex (TN); [3H]-Prazosin; [4-(4-amino-6,7-dimethoxy-quinazolin-2-yl)piperazin-1-yl]-(2-furyl)methanone; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](furan-2-yl)methanone; [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(furan-2-yl)methanone
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | High blood pressure [ICD11: BA00] | Approved | [1] | |||
| Severe congestive heart failure [ICD11: BD10] | Approved | [1] | ||||
| Therapeutic Class |
Antihypertensive Agents
|
|||||
| Structure |
|
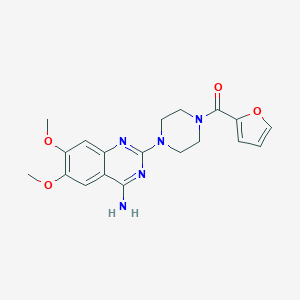 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H21N5O4
|
|||||
| Canonical SMILES |
COC1=C(C=C2C(=C1)C(=NC(=N2)N3CCN(CC3)C(=O)C4=CC=CO4)N)OC
|
|||||
| InChI |
InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22)
|
|||||
| InChIKey |
IENZQIKPVFGBNW-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 19216-56-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 383.4 | Topological Polar Surface Area | 107 | ||
| Heavy Atom Count | 28 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
11112649
, 11112650
, 11113367
, 11120245
, 11120733
, 11121221
, 11121703
, 11122183
, 11335550
, 11360789
, 11362790
, 11363716
, 11365352
, 11366278
, 11367914
, 11368840
, 11370831
, 11370832
, 11371821
, 11373515
, 11374104
, 11376076
, 11377002
, 11406810
, 11461761
, 11466975
, 11468095
, 11485033
, 11486809
, 11489105
, 11490367
, 11492298
, 11494636
, 14804776
, 26751613
, 26751614
, 29223971
, 4500854
, 46508594
, 47364941
, 47364942
, 47588782
, 47662032
, 47662033
, 47662034
, 47662035
, 7980376
, 8153011
, 841974
, 9572
|
|||||
| ChEBI ID |
ChEBI:8364
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | P-GP | Transporter Info | Km = 20 microM | High five cells-MDR1 | [4] | |
| References | ||||||
| 1 | Prazosin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | P-glycoprotein and breast cancer resistance protein expression and function at the blood-brain barrier and blood-cerebrospinal fluid barrier (choroid plexus) in streptozotocin-induced diabetes in rats. Brain Res. 2011 Jan 25;1370:238-45. | |||||
| 3 | Implications of genetic polymorphisms in drug transporters for pharmacotherapy. Cancer Lett. 2006 Mar 8;234(1):4-33. | |||||
| 4 | Evidence for two nonidentical drug-interaction sites in the human P-glycoprotein. Proc Natl Acad Sci U S A. 1997 Sep 30;94(20):10594-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
