Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01175
|
|||||
| Drug Name |
Didanosine
|
|||||
| Synonyms |
69655-05-6; 9-(2,3-Dideoxy-beta-D-ribofuranosyl)-6-oxopurine; 9-[(2R,5R)-5-(HYDROXYMETHYL)TETRAHYDROFURAN-2-YL]-1,9-DIHYDRO-6H-PURIN-6-ONE; 9-[(2R,5S)-5-(hydroxymethyl)tetrahydrofuran-2-yl]-1,9-dihydro-6H-purin-6-one; BMY 40900; BMY-40900; CHEBI:490877; DDI; DIDEOXYINOSINE; DRG-0016; DSSTox_CID_2927; Didanosina; Didanosina [INN-Spanish]; Didanosinum; Didanosinum [INN-Latin]; Inosine, 2',3'-dideoxy-; K3GDH6OH08; NCGC00090691-03; NCGC00159514-02; NSC 612049; UNII-K3GDH6OH08; Videx; Videx EC; ddIno; didanosine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Human immunodeficiency virus infection [ICD11: 1C62.Z] | Approved | [1] | |||
| Therapeutic Class |
Anti-HIV Agents
|
|||||
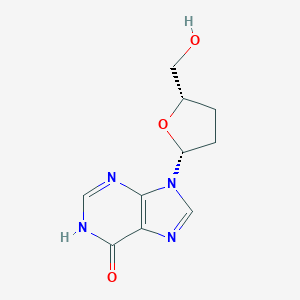
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C10H12N4O3
|
|||||
| Canonical SMILES |
C1CC(OC1CO)N2C=NC3=C2N=CNC3=O
|
|||||
| InChI |
InChI=1S/C10H12N4O3/c15-3-6-1-2-7(17-6)14-5-13-8-9(14)11-4-12-10(8)16/h4-7,15H,1-3H2,(H,11,12,16)/t6-,7+/m0/s1
|
|||||
| InChIKey |
BXZVVICBKDXVGW-NKWVEPMBSA-N
|
|||||
| CAS Number |
CAS 69655-05-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 236.23 | Topological Polar Surface Area | 88.7 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 5 | |||
| XLogP |
-1.2
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103529091
, 12014245
, 14847321
, 15121787
, 15121788
, 15196316
, 17389541
, 22431600
, 25819905
, 26719672
, 26754510
, 26757698
, 29215491
, 34715128
, 46386730
, 46506255
, 46508438
, 48415887
, 48422196
, 48422428
, 48423223
, 48424479
, 49681777
, 49718183
, 56463354
, 57313303
, 58717761
, 595889
, 596438
, 596681
, 597344
, 601538
, 602978
, 622273
, 77794640
, 7847362
, 7885163
, 7979070
, 7979071
, 81093234
, 833736
, 87351572
, 87559876
, 90451743
, 91612512
, 9168
, 92308421
, 92308956
, 92711314
, 92729668
|
|||||
| ChEBI ID |
CHEBI:490877
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | CNT2 | Transporter Info | Concentrative nucleoside transporter 2 | Substrate | [2] | |
| References | ||||||
| 1 | Didanosine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Clinical Nephrotoxins: Renal Injury from Drugs and Chemicals. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
