Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01195
|
|||||
| Drug Name |
Rabeprazole
|
|||||
| Synonyms |
2-(((4-(3-Methoxypropoxy)-3-methyl-2-pyridinyl)methyl)sulfinyl)-1H-benzimidazole; 2-((4-(3-methoxypropoxy)-3-methylpyridin-2-yl)methylsulfinyl)-1H-benzimidazole; 2-({[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methyl}sulfinyl)-1H-benzimidazole; 2-[[4-(3-methoxypropoxy)-3-methylpyridin-2-yl]methylsulfinyl]-1H-benzimidazole; Aciphex (TN); CL23619; Dexrabeprazole; Eraloc; Eraloc (TN); LY307640; Pariet (TN); Rabeprazole (INN); Rabeprazole [BAN:INN]; Rabeprazole [INN:BAN]; Rablet (TN); Rebeprazole sodium
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Gastroesophageal reflux disease [ICD11: DA22] | Approved | [1] | |||
| Peptic ulcer [ICD11: DA61] | Approved | [1] | ||||
| Therapeutic Class |
Antiulcer Agents
|
|||||
| Structure |
|
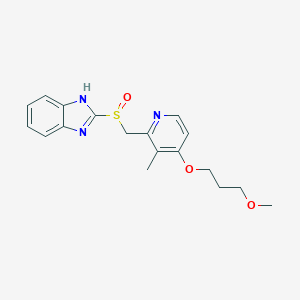 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C18H21N3O3S
|
|||||
| Canonical SMILES |
CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCCCOC
|
|||||
| InChI |
InChI=1S/C18H21N3O3S/c1-13-16(19-9-8-17(13)24-11-5-10-23-2)12-25(22)18-20-14-6-3-4-7-15(14)21-18/h3-4,6-9H,5,10-12H2,1-2H3,(H,20,21)
|
|||||
| InChIKey |
YREYEVIYCVEVJK-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 117976-89-3
|
|||||
| Pharmaceutical Properties | Molecular Weight | 359.4 | Topological Polar Surface Area | 96.3 | ||
| Heavy Atom Count | 25 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 1 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
1.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10066
, 103370406
, 104029616
, 104308073
, 118212884
, 124659135
, 125338815
, 126525321
, 126591137
, 126655846
, 126670688
, 126670689
, 129836330
, 131295768
, 134338014
, 135040318
, 137018746
, 142316515
, 142318546
, 142519546
, 14828218
, 152034678
, 160825170
, 160964463
, 162022428
, 163092473
, 163384417
, 164339280
, 164787748
, 166650031
, 170500544
, 172080531
, 178103864
, 179116873
, 29224100
, 46386636
, 46506366
, 49681560
, 49742702
, 50064224
, 50769860
, 5392621
, 57288826
, 57322572
, 7980480
, 8153096
, 85174502
, 92308674
, 93166177
, 96025149
|
|||||
| ChEBI ID |
ChEBI:8768
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| References | ||||||
| 1 | Rabeprazole was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol. 2009 Jan;67(1):44-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
