Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01203
|
|||||
| Drug Name |
Dactinomycin
|
|||||
| Synonyms |
1H-Pyrrolo(2,1-1)-(1,4,7,10,13)oxatetraazacyclohexadecine; 4,6-dimethyl-3-oxo-3H-phenoxazine-1,9-dicarboxamide; ACT D; ACTINOMYCIN D AMP; AD (VAN); Actactinomycin A IV; Actinomycin 11 cosmegen; Actinomycin 7; Actinomycin A IV; Actinomycin Aiv; Actinomycin C (sub1); Actinomycin C(sub1); Actinomycin C1; Actinomycin D (JP15); Actinomycin D deriv. of 3H-phenoxaocardazine; Actinomycin D, sodium deoxyribonucleic acid complex; Actinomycin I; Actinomycin I (sub1); Actinomycin I(sub 1); Actinomycin I(sub1); Actinomycin I1; Actinomycin IV; Actinomycin X 1; Actinomycin X1; Actinomycin cl; Actinomycin x i; Actinomycin-(threo-val-pro-sar-meval); Actinomycin-IV; Actinomycin-[threo-val-pro-sar-meval]; Actinomycindioic D acid, dilactone; Actinomyein-theo-val-pro-sar-meval; Acto-D; Antibiotic from Streptomyces parvullus; COSMEGEN (TN); Chounghwamycin B; Cosmegen; D Actinomycin; DVA-DPR-SAR-MVA-(c1)DTH-PXZ-(c11)DTH-DVA-DPR-SAR-MVA; Dactinomicina; Dactinomicina [INN-Spanish]; Dactinomycin (USP); Dactinomycin D; Dactinomycin [USAN:BAN]; Dactinomycine; Dactinomycine [INN-French]; Dactinomycinum; Dactinomycinum [INN-Latin]; Dactinomyein d; Dilactone actin omycindioic D acid; Dilactone actinomycin D acid; Dilactone actinomycindioic D acid; GNF-PF-1977; HBF 386; HBF 386 meractinomycin; Lyovac cosmegen; Meractinomycin; NP-005932; O)-(1-oxo-1,2-ethanediyl)]bis(N-methyl)L-valine; Oncostatin K; PXZ-THR-DVA-PRO-SAR-MVA-THR-DVA-PRO-SAR-MVA; X 97
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Cancer [ICD11: 2A00-2F9Z] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
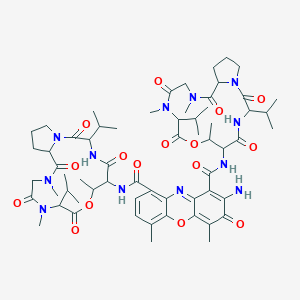 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C62H86N12O16
|
|||||
| Canonical SMILES |
CC1C(C(=O)NC(C(=O)N2CCCC2C(=O)N(CC(=O)N(C(C(=O)O1)C(C)C)C)C)C(C)C)NC(=O)C3=C4C(=C(C=C3)C)OC5=C(C(=O)C(=C(C5=N4)C(=O)NC6C(OC(=O)C(N(C(=O)CN(C(=O)C7CCCN7C(=O)C(NC6=O)C(C)C)C)C)C(C)C)C)N)C
|
|||||
| InChI |
InChI=1S/C62H86N12O16/c1-27(2)42-59(84)73-23-17-19-36(73)57(82)69(13)25-38(75)71(15)48(29(5)6)61(86)88-33(11)44(55(80)65-42)67-53(78)35-22-21-31(9)51-46(35)64-47-40(41(63)50(77)32(10)52(47)90-51)54(79)68-45-34(12)89-62(87)49(30(7)8)72(16)39(76)26-70(14)58(83)37-20-18-24-74(37)60(85)43(28(3)4)66-56(45)81/h21-22,27-30,33-34,36-37,42-45,48-49H,17-20,23-26,63H2,1-16H3,(H,65,80)(H,66,81)(H,67,78)(H,68,79)
|
|||||
| InChIKey |
RJURFGZVJUQBHK-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 50-76-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 1255.4 | Topological Polar Surface Area | 356 | ||
| Heavy Atom Count | 90 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 5 | Hydrogen Bond Acceptor Count | 18 | |||
| XLogP |
3.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103185659
, 103934097
, 104299382
, 11120223
, 11120711
, 11121199
, 11147306
, 11434891
, 124766124
, 124886988
, 125537037
, 125767589
, 126687205
, 131332481
, 134337828
, 137156344
, 137545216
, 160964308
, 161005131
, 162022425
, 162022855
, 164787451
, 174007348
, 26752119
, 29221206
, 3141480
, 47216557
, 47515096
, 47588774
, 47959487
, 48034857
, 48034858
, 49854365
, 50114308
, 51071907
, 520465
, 5343980
, 565029
, 57321102
, 57392818
, 74382088
, 75117937
, 7847281
, 8136899
, 8151387
, 841047
, 85083371
, 85789491
, 8990
, 95152991
|
|||||
| ChEBI ID |
ChEBI:27666
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP1 | Transporter Info | Multidrug resistance-associated protein 1 | Substrate | [3] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [4] | ||
| References | ||||||
| 1 | Actinomycin D was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol. 2003 Jan;63(1):65-72. | |||||
| 3 | Expression of multidrug resistance-associated protein in NIH/3T3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995 Nov 15;55(22):5342-7. | |||||
| 4 | Potential role of drug transporters in the pathogenesis of medically intractable epilepsy. Epilepsia. 2005 Feb;46(2):224-35. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
