Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01288
|
|||||
| Drug Name |
Palbociclib
|
|||||
| Synonyms |
LQQ; 571190-30-2; 6-ACETYL-8-CYCLOPENTYL-5-METHYL-2-[(5-PIPERAZIN-1-YLPYRIDIN-2-YL)AMINO]PYRIDO[2,3-D]PYRIMIDIN-7(8H)-ONE; 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-ylpyridin-2-ylamino)-8H-pyrido(2,3-d)pyrimidin-7-one; 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)pyridin-2-yl]amino]-8H-pyrido[2,3-d]pyrimidin-7-one; 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one; 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one; G9ZF61LE7G; HMR-2934; Ibrance; PD 0332991; PD 332991; PD 332991, PD 0332991, PD0332991; PD-0332991; PD-332991; PD0332991; Palbociclib(PD0332991); UNII-G9ZF61LE7G
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Breast cancer [ICD11: 2C60-2C6Z] | Approved | [1] | |||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
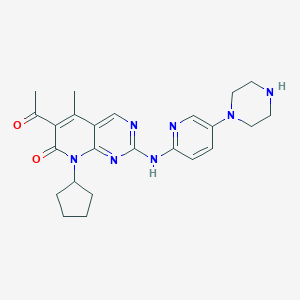 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C24H29N7O2
|
|||||
| Canonical SMILES |
CC1=C(C(=O)N(C2=NC(=NC=C12)NC3=NC=C(C=C3)N4CCNCC4)C5CCCC5)C(=O)C
|
|||||
| InChI |
InChI=1S/C24H29N7O2/c1-15-19-14-27-24(28-20-8-7-18(13-26-20)30-11-9-25-10-12-30)29-22(19)31(17-5-3-4-6-17)23(33)21(15)16(2)32/h7-8,13-14,17,25H,3-6,9-12H2,1-2H3,(H,26,27,28,29)
|
|||||
| InChIKey |
AHJRHEGDXFFMBM-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 571190-30-2
|
|||||
| Pharmaceutical Properties | Molecular Weight | 447.5 | Topological Polar Surface Area | 103 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
1.8
|
|||||
| PubChem CID | ||||||
| PubChem SID |
8035907
, 11062094
, 11538604
, 12015755
, 14808444
, 17137183
, 39302900
, 46394060
, 57361299
, 78833389
, 99436913
, 103461613
, 103905660
, 103905661
, 113915142
, 121280258
, 124360805
, 124757012
, 125164718
, 126580155
, 126671645
, 126737419
, 131480762
, 134339015
, 134339302
, 134339471
, 134964422
, 135262045
, 135686212
, 135686213
, 135686228
, 135686229
, 136367313
, 136367838
, 136920362
, 137184856
, 137275973
, 141483504
, 143498887
, 144116345
, 152240013
, 152258827
, 152344089
, 160647678
, 162202562
, 164045126
, 172232465
, 172919351
, 177748911
, 178103952
|
|||||
| ChEBI ID |
CHEBI:85993
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [2] | ||
| References | ||||||
| 1 | Palbociclib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Efflux transporters at the blood-brain barrier limit delivery and efficacy of cyclin-dependent kinase 4/6 inhibitor palbociclib (PD-0332991) in an orthotopic brain tumor model. J Pharmacol Exp Ther. 2015 Nov;355(2):264-71. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
