Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01330
|
|||||
| Drug Name |
Clofarabine
|
|||||
| Synonyms |
(2R,3R,4S,5R)-5-(6-amino-2-chloropurin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol; 2-Chloro-9-(2-deoxy-2-fluoro-b-D-arabinofuranosyl)-9H-purin-6-amine; 2-Chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)-9H-purin-6-amine; 2-Chloro-9-(2-deoxy-2-fluoro-beta-D-arabinofuranosyl)adenine; 2-Cl-2'-F-araA; 2-chloro-9-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)adenine; 3S211048; CAFdA; CFB; Cl-F-Ara-A; Clofarabina; Clofarabine (USAN/INN); Clofarabine [USAN]; Clofarabinum; Clofarex; Clolar; Clolar (TN); Clolar, Evoltra, Clofarabine; Evoltra; Evoltra (TN)
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acute lymphoblastic leukemia [ICD11: 2B33.0] | Approved | [1] | |||
| Juvenile myelomonocytic leukemia [ICD11: 2A42] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
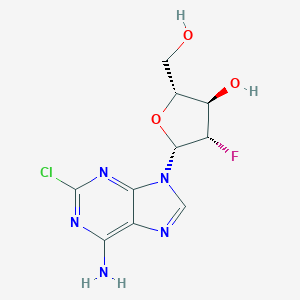 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C10H11ClFN5O3
|
|||||
| Canonical SMILES |
C1=NC2=C(N=C(N=C2N1C3C(C(C(O3)CO)O)F)Cl)N
|
|||||
| InChI |
InChI=1S/C10H11ClFN5O3/c11-10-15-7(13)5-8(16-10)17(2-14-5)9-4(12)6(19)3(1-18)20-9/h2-4,6,9,18-19H,1H2,(H2,13,15,16)/t3-,4+,6-,9-/m1/s1
|
|||||
| InChIKey |
WDDPHFBMKLOVOX-AYQXTPAHSA-N
|
|||||
| CAS Number |
CAS 123318-82-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 303.68 | Topological Polar Surface Area | 119 | ||
| Heavy Atom Count | 20 | Rotatable Bond Count | 2 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
0.9
|
|||||
| PubChem CID | ||||||
| PubChem SID |
8149071
, 10238376
, 10317422
, 12014412
, 14800615
, 14800616
, 29300120
, 46504968
, 47205855
, 50027432
, 56459308
, 57339361
, 71821361
, 77716344
, 81092867
, 92309003
, 92719465
, 93608050
, 99437031
, 99444025
, 103707445
, 104407717
, 109610765
, 118048878
, 124757091
, 125163895
, 126584329
, 126620778
, 126652234
, 126671067
, 129564367
, 134223319
, 134338128
, 135073562
, 135698306
, 136920392
, 136946661
, 136949087
, 137005624
, 141857409
, 143493352
, 144115841
, 144205735
, 152058774
, 152237732
, 152258941
, 160647786
, 160963976
, 162011506
, 162176704
|
|||||
| ChEBI ID |
CHEBI:681569
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| CNT3 | Transporter Info | Concentrative Na(+)-nucleoside cotransporter 3 | Substrate | [3] | ||
| ENT1 | Transporter Info | Equilibrative nucleoside transporter 1 | Substrate | [3] | ||
| ENT2 | Transporter Info | Equilibrative nucleoside transporter 2 | Substrate | [3] | ||
| References | ||||||
| 1 | Clofarabine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther. 2008 Sep;7(9):3092-102. | |||||
| 3 | Cytarabine-resistant leukemia cells are moderately sensitive to clofarabine in vitro. Anticancer Res. 2014 Apr;34(4):1657-62. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
