Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01377
|
|||||
| Drug Name |
Piritrexim
|
|||||
| Synonyms |
Piritrexim; 72732-56-0; Piritrexim [INN]; Piritreximum [Latin]; Piritrexime [French]; 6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine-2,4-diamine; Piritrexima [Spanish]; BW 301U; UNII-MK2A783ZUT; BW-301U; TCMDC-137235; BRN 5768301; MK2A783ZUT; CHEMBL7492; 2,4-Diamino-5-methyl-6-(2,5-dimethoxybenzyl)pyrido(2,3-d)pyrimidine; 6-(2,5-DIMETHOXY-BENZYL)-5-METHYL-PYRIDO[2,3-D]PYRIMIDINE-2,4-DIAMINE; 6-((2,5-Dimethoxyphenyl)methyl)-5-methylpyrido(2,3-d)pyrimidine-2,4-diamine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Urethral cancer [ICD11: 2F78] | Phase 2 | [1] | |||
| Structure |
|
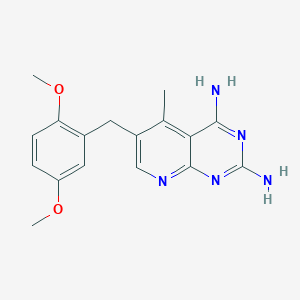 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C17H19N5O2
|
|||||
| Canonical SMILES |
CC1=C2C(=NC(=NC2=NC=C1CC3=C(C=CC(=C3)OC)OC)N)N
|
|||||
| InChI |
InChI=1S/C17H19N5O2/c1-9-11(6-10-7-12(23-2)4-5-13(10)24-3)8-20-16-14(9)15(18)21-17(19)22-16/h4-5,7-8H,6H2,1-3H3,(H4,18,19,20,21,22)
|
|||||
| InChIKey |
VJXSSYDSOJBUAV-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 72732-56-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 325.4 | Topological Polar Surface Area | 109 | ||
| Heavy Atom Count | 24 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 2 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
2.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
585461
, 596153
, 5321568
, 7889090
, 8183612
, 12012913
, 14801638
, 26704486
, 34718371
, 46506046
, 46518330
, 50035075
, 50293384
, 85866344
, 93579418
, 103165437
, 103855306
, 104304731
, 126420216
, 129485784
, 134340716
, 135029179
, 137008239
, 143157948
, 160966790
, 163724435
, 172896495
, 178103986
, 179151032
, 198977797
, 208012006
, 219402188
, 223518945
, 225249588
, 226399387
, 241132020
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| References | ||||||
| 1 | ClinicalTrials.gov (NCT00002914) Piritrexim in Treating Patients With Advanced Cancer of the Urinary Tract | |||||
| 2 | Mutant Gly482 and Thr482 ABCG2 mediate high-level resistance to lipophilic antifolates. Cancer Chemother Pharmacol. 2006 Dec;58(6):826-34. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
