Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01434
|
|||||
| Drug Name |
Trimetrexate
|
|||||
| Synonyms |
TMQ; Trimetrexato; Trimetrexatum; JB 11; Jb-11; Trimetrexato [INN-Spanish]; Trimetrexatum [INN-Latin]; Trimetrexate (USAN/INN); Trimetrexate [USAN:BAN:INN]; 2,4-Diamino-5-methyl-6-((3,4,5-trimethoxyanilino)methyl)quinazoline; 5-Methyl-6-(((3,4,5-trimethoxyphenyl)amino)methyl)-2,4-quinazolinediamine; 5-methyl-6-({[3,4,5-tris(methyloxy)phenyl]amino}methyl)quinazoline-2,4-diamine; 5-methyl-6-[(3,4,5-trimethoxyanilino)methyl]quinazoline-2,4-diamine; 6-[((3,4,5-Trimethoxyphenyl)amino)methyl]-5-methyl-2,4-quinazolinediamine
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Pneumocystis carinii pneumonia [ICD11: CA40.20] | Approved | [1] | |||
| Therapeutic Class |
Antifungal Agents
|
|||||
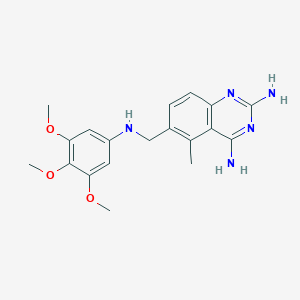
| Structure |
|
 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C19H23N5O3
|
|||||
| Canonical SMILES |
CC1=C(C=CC2=C1C(=NC(=N2)N)N)CNC3=CC(=C(C(=C3)OC)OC)OC
|
|||||
| InChI |
InChI=1S/C19H23N5O3/c1-10-11(5-6-13-16(10)18(20)24-19(21)23-13)9-22-12-7-14(25-2)17(27-4)15(8-12)26-3/h5-8,22H,9H2,1-4H3,(H4,20,21,23,24)
|
|||||
| InChIKey |
NOYPYLRCIDNJJB-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 52128-35-5
|
|||||
| Pharmaceutical Properties | Molecular Weight | 369.4 | Topological Polar Surface Area | 118 | ||
| Heavy Atom Count | 27 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 8 | |||
| XLogP |
2.5
|
|||||
| PubChem CID | ||||||
| PubChem SID |
13336
, 136953
, 596038
, 6646166
, 7980836
, 8153437
, 12012739
, 14803927
, 26755046
, 29224621
, 46505247
, 46518373
, 47207896
, 48416673
, 50064855
, 50110873
, 53787691
, 56435904
, 57322856
, 58106635
, 78747684
, 93166287
, 96021815
, 103181342
, 104309650
, 124892340
, 124892341
, 128733693
, 134337925
, 135000788
, 137002678
, 142433670
, 160964491
, 162973724
, 164784678
, 172916567
, 179117157
, 198941687
, 223365948
, 223366334
, 223773875
, 226396064
|
|||||
| ChEBI ID |
CHEBI:9737
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| References | ||||||
| 1 | Trimetrexate was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Mutant Gly482 and Thr482 ABCG2 mediate high-level resistance to lipophilic antifolates. Cancer Chemother Pharmacol. 2006 Dec;58(6):826-34. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
