Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01545
|
|||||
| Drug Name |
Pitavastatin
|
|||||
| Synonyms |
Pitavastatin; Itavastatin; Livalo; NK 104; Pitavastatin [INN]; Pitavastatin calcium; UNII-M5681Q5F9P; NK-104; C25H24FNO4; M5681Q5F9P; Zypitamag; Flovas; (3R,5S,6E)-7-(2-Cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl)-3,5-dihydroxyhept-6-enoic acid; P 872441; P-872441; (3R,5S,6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)quinolin-3-yl]-3,5-dihydroxyhept-6-enoic acid; ( )-(3R,5S,6E)-7-(2-Cyclopropyl-4-(4-fluorophenyl)-3-quinolyl)-3,5-dihydroxy-6-heptenoic acid; NK 104 (acid); Pitavastatin calcium (JAN)
|
|||||
| Indication | Primary hyperlipidemia and mixed dyslipidemia [ICD11: 5C8Z] | Approved | [1] | |||
| Structure |
|
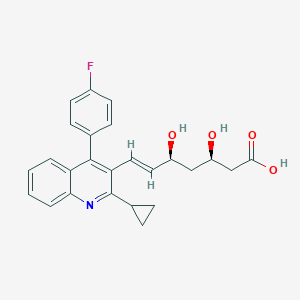 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C25H24FNO4
|
|||||
| Canonical SMILES |
C1CC1C2=NC3=CC=CC=C3C(=C2C=CC(CC(CC(=O)O)O)O)C4=CC=C(C=C4)F
|
|||||
| InChI |
InChI=1S/C25H24FNO4/c26-17-9-7-15(8-10-17)24-20-3-1-2-4-22(20)27-25(16-5-6-16)21(24)12-11-18(28)13-19(29)14-23(30)31/h1-4,7-12,16,18-19,28-29H,5-6,13-14H2,(H,30,31)/b12-11+/t18-,19-/m1/s1
|
|||||
| InChIKey |
VGYFMXBACGZSIL-MCBHFWOFSA-N
|
|||||
| CAS Number |
CAS 147511-69-1
|
|||||
| Pharmaceutical Properties | Molecular Weight | 421.5 | Topological Polar Surface Area | 90.6 | ||
| Heavy Atom Count | 31 | Rotatable Bond Count | 8 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
3.5
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:32020
|
|||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OATP1A2 | Transporter Info | Organic anion transporting polypeptide 1A2 | Substrate | [4] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [5] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [6] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [7] | ||
| References | ||||||
| 1 | Pitavastatin was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | ABCG2: a perspective. Adv Drug Deliv Rev. 2009 Jan 31;61(1):3-13. | |||||
| 3 | Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005 Sep;68(3):800-7. | |||||
| 4 | Transporter-mediated influx and efflux mechanisms of pitavastatin, a new inhibitor of HMG-CoA reductase. J Pharm Pharmacol. 2005 Oct;57(10):1305-11. | |||||
| 5 | Prediction of the in vivo OATP1B1-mediated drug-drug interaction potential of an investigational drug against a range of statins. Eur J Pharm Sci. 2012 Aug 30;47(1):244-55. | |||||
| 6 | Impact of OATP transporters on pharmacokinetics. Br J Pharmacol. 2009 Oct;158(3):693-705. | |||||
| 7 | Intestinal absorption of HMG-CoA reductase inhibitor pitavastatin mediated by organic anion transporting polypeptide and P-glycoprotein/multidrug resistance 1. Drug Metab Pharmacokinet. 2011;26(2):171-9. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
