Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01619
|
|||||
| Drug Name |
BMS 536924
|
|||||
| Synonyms |
BMS-536924; BMS 536924; BMS536924; UNII-40E3AZG1MX; 4-[[(2S)-2-(3-Chlorophenyl)-2-hydroxyethyl]amino]-3-[7-methyl-5-(4-morpholinyl)-1H-benzimidazol-2-yl]-2(1H)-pyridinone; 40E3AZG1MX; CHEMBL401930; CS-0117; HY-10262; MLS006011171; SCHEMBL4132577; SCHEMBL15974144; BDBM27879; BCP02116; ZINC6718468; 2278AH; s1012; ABP000159; ZINC140935730; AKOS024458339; AKOS025149514; SB19378; RL03740; KIN0000061; NCGC00346460-05; NCGC00346460-02; SMR004702940; BMS-536924/BMS536924; A2238; X5078; SW218124-2; S-7752
|
|||||
| Indication | Cancer [ICD11: 2A00-2F9Z] | Preclinical | [1] | |||
| Structure |
|
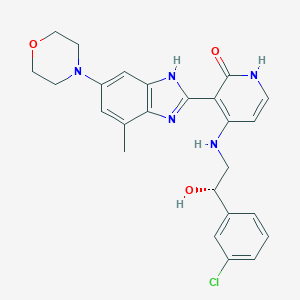 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C25H26ClN5O3
|
|||||
| Canonical SMILES |
CC1=CC(=CC2=C1N=C(N2)C3=C(C=CNC3=O)NCC(C4=CC(=CC=C4)Cl)O)N5CCOCC5
|
|||||
| InChI |
InChI=1S/C25H26ClN5O3/c1-15-11-18(31-7-9-34-10-8-31)13-20-23(15)30-24(29-20)22-19(5-6-27-25(22)33)28-14-21(32)16-3-2-4-17(26)12-16/h2-6,11-13,21,32H,7-10,14H2,1H3,(H,29,30)(H2,27,28,33)/t21-/m1/s1
|
|||||
| InChIKey |
ZWVZORIKUNOTCS-OAQYLSRUSA-N
|
|||||
| CAS Number |
CAS 468740-43-4
|
|||||
| Pharmaceutical Properties | Molecular Weight | 480 | Topological Polar Surface Area | 103 | ||
| Heavy Atom Count | 34 | Rotatable Bond Count | 6 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 6 | |||
| XLogP |
2.7
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:91454
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| References | ||||||
| 1 | National Center for Advancing Translational Science-Inxight: drug (40E3AZG1MX) | |||||
| 2 | Drug efflux by breast cancer resistance protein is a mechanism of resistance to the benzimidazole insulin-like growth factor receptor/insulin receptor inhibitor, BMS-536924. Mol Cancer Ther. 2011 Jan;10(1):117-25. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
