Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01640
|
|||||
| Drug Name |
Scutellarin
|
|||||
| Synonyms |
Scutellarin; Breviscapin; Scutellarein-7-glucuronide; Scutellarin B; Breviscapine; Scutellarein-7beta-D-glucuronide; Scutellarein 7-beta-D-glucuronide; Scutellarein-7beta-D-glucuronoside; Scutellarein-7-O-beta-D-glucuronide; UNII-16IGP0ML9A; Scutellarein 7-b-D-glucuronide; Scutellarein 7-O-beta-D-glucuronide; 16IGP0ML9A; SCUTELLAREIN 7-O-GLUCURONIDE; beta-D-Glucopyranosiduronic acid, 5,6-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-1-benzopyran-7-yl
|
|||||
| Indication | Cerebrovascular ischaemia [ICD11: 8B1Z] | Preclinical | [1] | |||
| Structure |
|
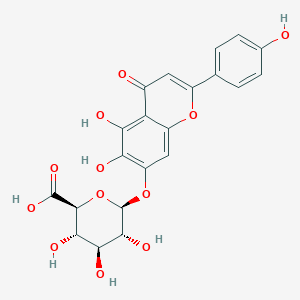 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H18O12
|
|||||
| Canonical SMILES |
C1=CC(=CC=C1C2=CC(=O)C3=C(C(=C(C=C3O2)OC4C(C(C(C(O4)C(=O)O)O)O)O)O)O)O
|
|||||
| InChI |
InChI=1S/C21H18O12/c22-8-3-1-7(2-4-8)10-5-9(23)13-11(31-10)6-12(14(24)15(13)25)32-21-18(28)16(26)17(27)19(33-21)20(29)30/h1-6,16-19,21-22,24-28H,(H,29,30)/t16-,17-,18+,19-,21+/m0/s1
|
|||||
| InChIKey |
DJSISFGPUUYILV-ZFORQUDYSA-N
|
|||||
| CAS Number |
CAS 27740-01-8
|
|||||
| Pharmaceutical Properties | Molecular Weight | 462.4 | Topological Polar Surface Area | 203 | ||
| Heavy Atom Count | 33 | Rotatable Bond Count | 4 | |||
| Hydrogen Bond Donor Count | 7 | Hydrogen Bond Acceptor Count | 12 | |||
| XLogP |
0.8
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:61278
|
|||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [2] | ||
| MRP3 | Transporter Info | Multidrug resistance-associated protein 3 | Substrate | [2] | ||
| OATP2B1 | Transporter Info | Organic anion transporting polypeptide 2B1 | Substrate | [2] | ||
| References | ||||||
| 1 | Scutellarin blocks sodium current in freshly isolated mouse hippocampal CA1 neurons. Neurochem Res. 2011 Jun;36(6):947-54. | |||||
| 2 | Mechanistic studies on the absorption and disposition of scutellarin in humans: selective OATP2B1-mediated hepatic uptake is a likely key determinant for its unique pharmacokinetic characteristics. Drug Metab Dispos. 2012 Oct;40(10):2009-20. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
