Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR01712
|
|||||
| Drug Name |
Barasertib-hQPA
|
|||||
| Synonyms |
AZD1152-HQPA; Barasertib (AZD1152-HQPA); AZD-1152HQPA; barasertib-hQPA; UNII-29P8LWS24N; INH 34; AZD1152 HPQA; 29P8LWS24N; CHEMBL215152; 2-[5-(7-{3-[Ethyl-(2-hydroxy-ethyl)-amino]-propoxy}-quinazolin-4-ylamino)-2H-pyrazol-3-yl]-N-(3-fluoro-phenyl)-acetamide; 2-(3-((7-(3-(ethyl(2-hydroxyethyl)amino)propoxy)quinazolin-4-yl)amino)-1H-pyrazol-5-yl)-N-(3-fluorophenyl)acetamide; 5-[[7-[3-[Ethyl(2-hydroxyethyl)amino]propoxy]-4-quinazolinyl]amino]-N-(3-fluorophenyl)-1H-pyrazole-3-acetamide; AZD 1152
|
|||||
| Indication | Cancer [ICD11: 2A00-2F9Z] | Preclinical | [1] | |||
| Structure |
|
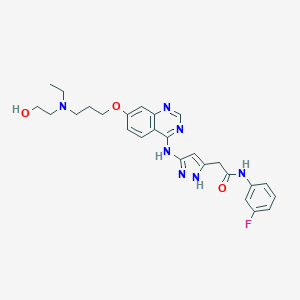 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C26H30FN7O3
|
|||||
| Canonical SMILES |
CCN(CCCOC1=CC2=C(C=C1)C(=NC=N2)NC3=NNC(=C3)CC(=O)NC4=CC(=CC=C4)F)CCO
|
|||||
| InChI |
InChI=1S/C26H30FN7O3/c1-2-34(10-11-35)9-4-12-37-21-7-8-22-23(16-21)28-17-29-26(22)31-24-14-20(32-33-24)15-25(36)30-19-6-3-5-18(27)13-19/h3,5-8,13-14,16-17,35H,2,4,9-12,15H2,1H3,(H,30,36)(H2,28,29,31,32,33)
|
|||||
| InChIKey |
QYZOGCMHVIGURT-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 722544-51-6
|
|||||
| Pharmaceutical Properties | Molecular Weight | 507.6 | Topological Polar Surface Area | 128 | ||
| Heavy Atom Count | 37 | Rotatable Bond Count | 13 | |||
| Hydrogen Bond Donor Count | 4 | Hydrogen Bond Acceptor Count | 9 | |||
| XLogP |
3
|
|||||
| PubChem CID | ||||||
| ChEBI ID |
CHEBI:91367
|
|||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| References | ||||||
| 1 | MedchemExpress: AZD1152-HQPA | |||||
| 2 | P-glycoprotein and breast cancer resistance protein in acute myeloid leukaemia cells treated with the aurora-B kinase inhibitor barasertib-hQPA. BMC Cancer. 2011 Jun 16;11:254. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
