Crystal Structure of This DT
Crystallisation Method: EM
Corresponding Chain: A
Sequence Length: 1-1480
Detail: Struture Info
| General Information of Drug Transporter (DT) | |||||
|---|---|---|---|---|---|
| DT ID | DTD0060 Transporter Info | ||||
| Gene Name | ABCC7 | ||||
| Protein Name | Cystic fibrosis transmembrane conductance regulator | ||||
| Gene ID | |||||
| UniProt ID | |||||
| 3D Structure |
Crystal Structure of This DT Crystallisation Method: EM Corresponding Chain: A Sequence Length: 1-1480 Detail: Struture Info |
||||
| Inter-species Structural Differences (ISD) | |||||
| Mus musculus (Mouse) | |||||
| Gene Name | Cftr | ||||
| UniProt ID | |||||
| UniProt Entry | |||||
| 3D Structure | |||||
| Click to Save PDB File in PDB Format | |||||
| Experimental Structures of This Model Oganism | |||||
| PDB ID | Scanning Method | Resolution | Expression System | Details | Ref |
| 1Q3H | X-ray | 2.5 Å | Escherichia coli | [ 1] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B/C/D | ||||
| Sequence Length | 389-673 | Mutation | No | ||
| 1R0X | X-ray | 2.2 Å | Escherichia coli | [ 1] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B/C/D | ||||
| Sequence Length | 389-673 | Mutation | No | ||
| 1R0Y | X-ray | 2.55 Å | Escherichia coli | [ 1] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B/C/D | ||||
| Sequence Length | 389-673 | Mutation | No | ||
| 1R0Z | X-ray | 2.35 Å | Escherichia coli | [ 1] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B/C/D | ||||
| Sequence Length | 389-673 | Mutation | Yes | ||
| 1R10 | X-ray | 3 Å | Escherichia coli | [ 1] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B | ||||
| Sequence Length | 389-673 | Mutation | No | ||
| 1XF9 | X-ray | 2.7 Å | Escherichia coli | [ 2] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B/C/D | ||||
| Sequence Length | 389-670 | Mutation | Yes | ||
| 1XFA | X-ray | 3.1 Å | Escherichia coli | [ 2] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B | ||||
| Sequence Length | 389-670 | Mutation | Yes | ||
| 3SI7 | X-ray | 2.25 Å | Escherichia coli | [ 3] | |
| Structure | |||||
| Click to Save PDB File in TXT Format | |||||
| Corresponding chain | A/B/C/D | ||||
| Sequence Length | 389-673 | Mutation | No | ||
| Inter-species Structural Differences (ISD) | |||||
| Rattus norvegicus (Rat) | |||||
| Gene Name | Cftr | ||||
| UniProt ID | |||||
| UniProt Entry | |||||
| 3D Structure |
Method: Homology modeling Teplate PDB: 5UAK_A Sequence Length: 1476 Identity: 77.92% |
||||
| Click to Save PDB File in TXT Format | |||||
| Performance | Minimized Score | -3200.166 kcal/mol | |||
| Ramachandra Favored |
Medium |
||||
| QMEANBrane Quality |
Medium |
||||
| Ramachandran Plot |
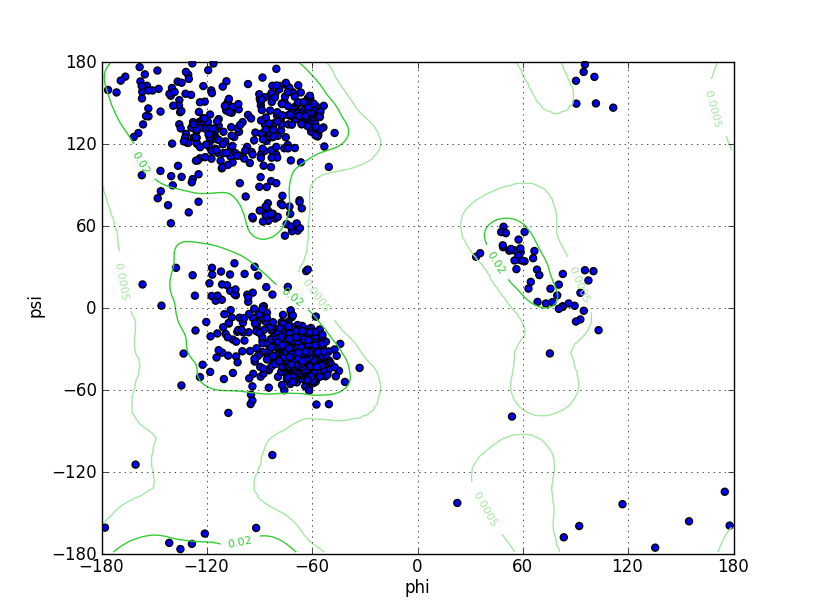
Ramz Z Score: -1.19 ± 0.2 Residues in Favored Region: 1427 Ramachandran favored: 96.81% Number of Outliers: 11 Ramachandran outliers: 0.75% |
||||
| Click to Save Ramachandran Plot in PNG Format | |||||
| Local Quality |

QMEANBrane Score: 0.75 |
||||
| Click to Save Local Quality Plot in PNG Format | |||||
| Inter-species Structural Differences (ISD) | |||||
| Oryctolagus cuniculus (Rabbit) | |||||
| Gene Name | CFTR | ||||
| UniProt ID | |||||
| UniProt Entry | |||||
| 3D Structure |
Method: Homology modeling Teplate PDB: 5UAK_A Sequence Length: 1481 Identity: 92.235% |
||||
| Click to Save PDB File in TXT Format | |||||
| Performance | Minimized Score | -3338.966 kcal/mol | |||
| Ramachandra Favored |
Medium |
||||
| QMEANBrane Quality |
Medium |
||||
| Ramachandran Plot |

Ramz Z Score: -1.26 ± 0.2 Residues in Favored Region: 1441 Ramachandran favored: 97.43% Number of Outliers: 5 Ramachandran outliers: 0.34% |
||||
| Click to Save Ramachandran Plot in PNG Format | |||||
| Local Quality |

QMEANBrane Score: 0.75 |
||||
| Click to Save Local Quality Plot in PNG Format | |||||
| References | |||||
| 1 | Structure of nucleotide-binding domain 1 of the cystic fibrosis transmembrane conductance regulator. EMBO J. 2004 Jan 28;23(2):282-93. | ||||
| 2 | Side chain and backbone contributions of Phe508 to CFTR folding. Nat Struct Mol Biol. 2005 Jan;12(1):10-6. | ||||
| 3 | Requirements for efficient correction of F508 CFTR revealed by analyses of evolved sequences. Cell. 2012 Jan 20;148(1-2):164-74. | ||||
| 4 | Conformational Changes of CFTR upon Phosphorylation and ATP Binding. Cell. 2017 Jul 27;170(3):483-491.e8. | ||||
| 5 | Cystic fibrosis transmembrane conductance regulator: solution structures of peptides based on the Phe508 region, the most common site of disease-causing DeltaF508 mutation. Biochemistry. 1999 Jun 8;38(23):7453-61. | ||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.