Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00304
|
|||||
| Drug Name |
Sorafenib
|
|||||
| Synonyms |
4(4-{3-[4-Chloro-3-(trifluoromethyl)phenyl]ureido}phenoxy)-N(sup 2)-methylpyridine-2-carboxamide; 4-(4-((((4-Chloro-3-(trifluoromethyl)phenyl)amino)carbonyl)amino)phenoxy)-N-methyl-2-pyridinecarboxamide; 4-(4-(3-(4-chloro-3-trifluoromethylphenyl)ureido)phenoxy)pyridine-2-carboxyllic acid methyamide-4-methylbenzenesulfonate; 4-(4-{3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido}phenoxy)-N(sup 2)-methylpyridine-2-carboxamide; 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-N-methylpyridine-2-carboxamide; 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methyl-pyridine-2-carboxamide; 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methylpyridine-2-carboxamide; 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide; 4-{4-[({[4-CHLORO-3-(TRIFLUOROMETHYL)PHENYL]AMINO}CARBONYL)AMINO]PHENOXY}-N-METHYLPYRIDINE-2-CARBOXAMIDE; N-(4-Chloro-3-(trifluoromethyl)phenyl)-N'-(4-(2-(N-methylcarbamoyl)-4-pyridyloxy)phenyl)urea; N-(4-chloro-3-(trifluoromethyl)phenyl)-N'-(4-(2-(N-methylcar bamoyl)-4-pyridyloxy)phenyl)urea; N-[4-Chloro-3-(trifluoromethyl)phenyl]-N'-[4-[2-(N-methylcarbamoyl)-4-pyridyloxy]phenyl]urea; Nexavar; Nexavar (TN); Sorafenib (INN); Sorafenib (Pan-TK inhibitor); Sorafenib [INN]; Sorafenibum
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Hepatocellular carcinoma [ICD11: 2C12.02] | Approved | [1] | |||
| Renal cell carcinoma [ICD11: 2C90] | Approved | [1] | ||||
| Differentiated thyroid cancer [ICD11: 2D10] | Approved | [1] | ||||
| Pancreatic cancer [ICD11: 2C10] | Approved | [1] | ||||
| Therapeutic Class |
Anticancer Agents
|
|||||
| Structure |
|
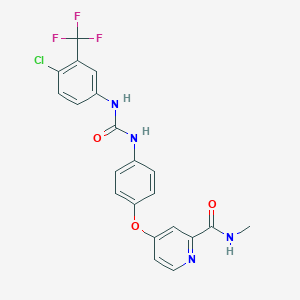 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C21H16ClF3N4O3
|
|||||
| Canonical SMILES |
CNC(=O)C1=NC=CC(=C1)OC2=CC=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F
|
|||||
| InChI |
InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31)
|
|||||
| InChIKey |
MLDQJTXFUGDVEO-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 284461-73-0
|
|||||
| Pharmaceutical Properties | Molecular Weight | 464.8 | Topological Polar Surface Area | 92.4 | ||
| Heavy Atom Count | 32 | Rotatable Bond Count | 5 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 7 | |||
| XLogP |
4.1
|
|||||
| PubChem CID | ||||||
| PubChem SID |
103420820
, 103904444
, 104178872
, 113461200
, 117695450
, 117871124
, 12015507
, 124767621
, 124893320
, 124893321
, 124893322
, 124893323
, 124893324
, 125346960
, 14720365
, 14834034
, 30419984
, 46505329
, 46516901
, 50069824
, 50070560
, 50071324
, 50100118
, 50112741
, 53787819
, 53799234
, 56312334
, 56312336
, 56312338
, 56312517
, 56312519
, 56312521
, 56394953
, 57399755
, 68529952
, 74382940
, 75518900
, 7886069
, 81092852
, 833589
, 833590
, 85174603
, 85285882
, 85845166
, 91148447
, 92718861
, 93581025
, 9372814
, 96025209
, 99443909
|
|||||
| ChEBI ID |
ChEBI:50924
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MRP2 | Transporter Info | Multidrug resistance-associated protein 2 | Substrate | [3] | ||
| OATP1B1 | Transporter Info | Organic anion transporting polypeptide 1B1 | Substrate | [4] | ||
| OATP1B3 | Transporter Info | Organic anion transporting polypeptide 1B3 | Substrate | [4] | ||
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [5] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [6] | ||
| RALBP1 | Transporter Info | RalBP1-associated Eps domain-containing protein 2 | Substrate | [7] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | RALBP1 | Transporter Info | Km = 10.4 microM | Human kidney cancer cells (Caki-2)-RALBP1 | [7] | |
| References | ||||||
| 1 | Sorafenib was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Double-transduced MDCKII cells to study human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) interplay in drug transport across the blood-brain barrier. Mol Pharm. 2011 Apr 4;8(2):571-82. | |||||
| 3 | Multidrug resistance protein 2 implicates anticancer drug-resistance to sorafenib. Biol Pharm Bull. 2011;34(3):433-5. | |||||
| 4 | Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013 Mar 15;19(6):1458-66. | |||||
| 5 | Perceived parental guan and school adjustment among Chinese early adolescents: The moderating role of interdependent self-construal. J Adolesc. 2019 Feb;71:18-27. | |||||
| 6 | Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010 Feb;9(2):319-26. | |||||
| 7 | Rlip76 transports sunitinib and sorafenib and mediates drug resistance in kidney cancer. Int J Cancer. 2010 Mar 15;126(6):1327-38. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
