Drug Information
| General Information | ||||||
|---|---|---|---|---|---|---|
| Drug ID |
DR00037
|
|||||
| Drug Name |
Cimetidine
|
|||||
| Synonyms |
1-Cyano-2-methyl-3-(2-(((5-methyl-4-imidazolyl)methyl)thio)ethyl)guanidine; 1-Cyano-2-methyl-3-[2-[[(5-methylimidazol-4-yl)methyl]thio]ethyl]guanidine; 1-cyano-2-methyl-3-[2-[(5-methyl-1H-imidazol-4-yl)methylsulfanyl]ethyl]guanidine; 2-Cyano-1-methyl-3-(2-(((5-methylimidazol-4-yl)methyl)thio)ethyl)guanidine; 2-Cyano-1-methyl-3-[2-(5-methyl-1H-imidazol-4-yl-methylthio)ethyl]guanidine; 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl}ethyl)guanidine; 2-cyano-1-methyl-3-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; Acibilin; Acinil; Altramet; Biomet400; Brumetidina; C 4522; CIMETIDINE A/AB; Ci metum; Cimal; Cimetadine; Cimetag; Cimetidina; Cimetidina [INN-Spanish]; Cimetidine (JP15/USP/INN); Cimetidine Hcl; Cimetidine [USAN:INN:BAN:JAN]; Cimetidinum; Cimetidinum [INN-Latin]; Cimetum; DRG-0150; Dyspamet; Edalene; Eureceptor; Evicer; FPF 1002; Gastrobitan; Gastromet; Histodil; Magicul; Metracin; N''-Cyano-N-methyl-N'-[2-[(5-methyl-1H-imidazol-4-yl)methylthio]ethyl]guanidine; N''-cyano-N-methyl-N'-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)-ethyl)guanidine; N''-cyano-N-methyl-N'-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; N-Cyano-N'-Methyl-N''-(2-(((5-Methyl-1H-Imidazol-4-YL)Methyl)Thio)Ethyl) Guanidine; N-Cyano-N'-methyl-N''-(2-(((5-methyl-1 H-imidazol-4-yl) methyl)thio)ethyl)guanidine; N-Cyano-N'-methyl-N''-(2-(((5-methyl-1H-imidazol-4-yl)methyl)thio)ethyl)guanidine; N-Cyano-N'-methyl-[2-[[[5-methyl-1H-imidazol-4-yl]methyl]thio]ethyl]guanidine; N-cyano-N'-methyl-N''-(2-([(5-methyl-1H-imidazol-4-yl)methyl]sulfanyl)ethyl)guanidine; N-cyano-N'-methyl-N''-(2-{[(5-methyl-1H-imidazol-4-yl)methyl]thio}ethyl)guanidine; Peptol; SK&F-92334; SKF 92334; SKF-92334; Sigmetadine; Tagamet; Tagamet (TN); Tagamet HB (TN); Tagamet HB200 (TN); Tagamet Hb; Tagamet Hb 200; Tagamet, SKF-92334, Tratul, Tametin, Dyspamet, Acinil, Cimetidine; Tametin; Tratul; Ulcedin; Ulcedine; Ulcestop; Ulcimet; Ulcofalk; Ulcomedina; Ulcomet; Ulhys; Valmagen; Venopex
|
|||||
| Drug Type |
Small molecular drug
|
|||||
| Indication | Acid reflux disorder [ICD11: DA22] | Approved | [1] | |||
| Therapeutic Class |
Antiulcer Agents
|
|||||
| Structure |
|
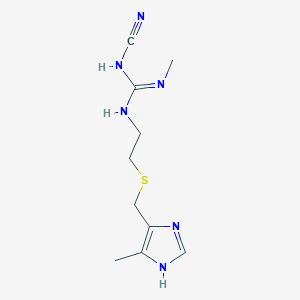 |
||||
| 3D MOL | 2D MOL | |||||
| Formula |
C10H16N6S
|
|||||
| Canonical SMILES |
CC1=C(N=CN1)CSCCNC(=NC)NC#N
|
|||||
| InChI |
InChI=1S/C10H16N6S/c1-8-9(16-7-15-8)5-17-4-3-13-10(12-2)14-6-11/h7H,3-5H2,1-2H3,(H,15,16)(H2,12,13,14)
|
|||||
| InChIKey |
AQIXAKUUQRKLND-UHFFFAOYSA-N
|
|||||
| CAS Number |
CAS 51481-61-9
|
|||||
| Pharmaceutical Properties | Molecular Weight | 252.34 | Topological Polar Surface Area | 114 | ||
| Heavy Atom Count | 17 | Rotatable Bond Count | 7 | |||
| Hydrogen Bond Donor Count | 3 | Hydrogen Bond Acceptor Count | 4 | |||
| XLogP |
0.4
|
|||||
| PubChem CID | ||||||
| PubChem SID |
10321166
, 10524846
, 11110948
, 11113773
, 11335341
, 11360580
, 11363389
, 11365951
, 11368513
, 11372297
, 11374462
, 11376675
, 11407328
, 11461552
, 11484620
, 11488598
, 11491204
, 11492627
, 11494309
, 11533056
, 12013445
, 14774387
, 15122273
, 15196790
, 17389962
, 17404856
, 22391437
, 24277766
, 24531048
, 26612025
, 26679798
, 26747359
, 26747360
, 26751956
, 26751957
, 29221911
, 4266417
, 4493559
, 460110
, 46487919
, 46505360
, 47275096
, 615111
, 6699439
, 6897918
, 7847361
, 7978946
, 8147032
, 8149633
, 9167
|
|||||
| ChEBI ID |
ChEBI:3699
|
|||||
| TTD Drug ID | ||||||
| DT(s) Transporting This Drug | BCRP | Transporter Info | Breast cancer resistance protein | Substrate | [2] | |
| MATE1 | Transporter Info | Multidrug and toxin extrusion protein 1 | Substrate | [3] | ||
| MATE2 | Transporter Info | Multidrug and toxin extrusion protein 2 | Substrate | [3] | ||
| OAT3 | Transporter Info | Organic anion transporter 3 | Substrate | [4] | ||
| OCT-1 | Transporter Info | Organic cation transporter 1 | Substrate | [5] | ||
| OCT-2 | Transporter Info | Organic cation transporter 2 | Substrate | [6] | ||
| P-GP | Transporter Info | P-glycoprotein 1 | Substrate | [7] | ||
| Drug-Transporter Activity Data | ||||||
| Drug-Transporter Activity Data | MATE1 | Transporter Info | Km = 170 microM | Human embryonic kidney cells (HEK293)-MATE1 | [3] | |
| MATE2 | Transporter Info | Km = 120 microM | Human embryonic kidney cells (HEK293)-MATE2K | [3] | ||
| MATE2 | Transporter Info | Km = 370 microM | Human embryonic kidney cells (HEK293)-MATE2K | [8] | ||
| OAT3 | Transporter Info | Km = 174 microM | Chinese hamster ovary (CHO) cells-OAT3 | [4] | ||
| OAT3 | Transporter Info | Km = 113 microM | Human embryonic kidney cells (HEK293)-OAT3 | [9] | ||
| OAT3 | Transporter Info | Km = 149 microM | Human embryonic kidney cells (HEK293)-OAT3 | [10] | ||
| OAT3 | Transporter Info | Km = 57.4 microM | Oocytes-OAT3 | [11] | ||
| OCT-2 | Transporter Info | Km = 60 microM | Human embryonic kidney cells (HEK293)-OCT2 | [5] | ||
| OCT-2 | Transporter Info | Km = 72.6 microM | Human embryonic kidney cells (HEK293)-OCT2 | [12] | ||
| References | ||||||
| 1 | Cimetidine was approved by FDA. The official website of the U.S. Food and Drug Administration. (2019) | |||||
| 2 | Progesterone acts via progesterone receptors A and B to regulate breast cancer resistance protein expression. Mol Pharmacol. 2008 Mar;73(3):613-5. | |||||
| 3 | Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007 Jul 15;74(2):359-71. | |||||
| 4 | Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007 May;321(2):673-83. | |||||
| 5 | Identification of novel substrates and structure-activity relationship of cellular uptake mediated by human organic cation transporters 1 and 2. J Med Chem. 2013 Sep 26;56(18):7232-42. | |||||
| 6 | Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab Pharmacokinet. 2010;25(4):328-34. | |||||
| 7 | Mammalian drug efflux transporters of the ATP binding cassette (ABC) family in multidrug resistance: A review of the past decade. Cancer Lett. 2016 Jan 1;370(1):153-64. | |||||
| 8 | Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006 Aug;17(8):2127-35. | |||||
| 9 | Inhibition of oat3-mediated renal uptake as a mechanism for drug-drug interaction between fexofenadine and probenecid. Drug Metab Dispos. 2006 May;34(5):743-7. | |||||
| 10 | Is the monkey an appropriate animal model to examine drug-drug interactions involving renal clearance? Effect of probenecid on the renal elimination of H2 receptor antagonists. J Pharmacol Exp Ther. 2006 Mar;316(3):1187-94. | |||||
| 11 | Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001 May;59(5):1277-86. | |||||
| 12 | A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J Pharmacol Exp Ther. 2005 Oct;315(1):337-45. | |||||
If you find any error in data or bug in web service, please kindly report it to Dr. Yin and Dr. Li.
